- NEWS AND VIEWS
The first synthetic element
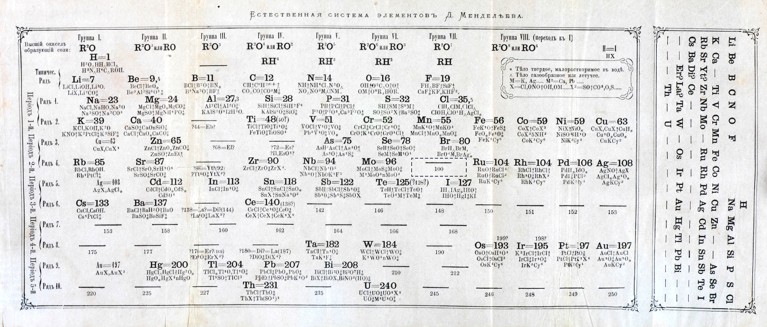
Figure 1 | Mendeleev’s periodic table. When Mendeleev devised his periodic table 150 years ago, he left spaces for elements that he thought were missing. The gap indicated by the dashed box is for element 43. Carlo Perrier and Emilio Segrè3 discovered this element, now known as technetium, in 1937. Credit: Science History Institute
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 565, 570-571 (2019)
doi: https://doi.org/10.1038/d41586-019-00236-4
References
Fermi, L. Atoms in the Family: My Life with Enrico Fermi (Univ. Chicago Press, 1954).
Fermi, E. et al. Nature 133, 898–899 (1934).
Perrier, C. & Segrè, E. Nature 140, 193–194 (1937).
Scerri, E. A Tale of Seven Elements (Oxford Univ. Press, 2013).
Segrè, E. & Seaborg, G. T. Phys. Rev. 54, 772 (1938).
Hahn, O. & Strassmann, F. Naturwissenschaften 27, 11–15 (1939).
Perrier, C. & Segrè, E. Nature 159, 24 (1947).
Segrè, E. Phys. Rev. 55, 1104 (1939).

 Anniversary celebrations are due for Mendeleev’s periodic table
Anniversary celebrations are due for Mendeleev’s periodic table
 Extreme chemistry: experiments at the edge of the periodic table
Extreme chemistry: experiments at the edge of the periodic table
 Can quantum ideas explain chemistry’s greatest icon?
Can quantum ideas explain chemistry’s greatest icon?
 Celebrate the women behind the periodic table
Celebrate the women behind the periodic table
 More than 2,000 years of elements: a prehistory of the periodic table
More than 2,000 years of elements: a prehistory of the periodic table
 In his element: looking back on Primo Levi’s The Periodic Table
In his element: looking back on Primo Levi’s The Periodic Table
 Futures SF: Elementary school
Futures SF: Elementary school