- NEWS AND VIEWS
Metabolism reprogrammed by the nitric oxide signalling molecule
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 565, 33-34 (2019)
doi: https://doi.org/10.1038/d41586-018-07457-z
References
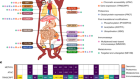
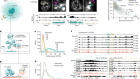
Zhou, H.-L. et al. Nature 565, 96–100 (2019).
Nathan, C. FASEB J. 6, 3051–3064 (1992).
Lowenstein, C. J. & Snyder, S. H. Cell 70, 705–707 (1992).
Maron, B. A., Tang, S.-S. & Loscalzo, J. Antioxidants Redox Signal. 18, 270–287 (2013).
Stamler, J. S. et al. Proc. Natl Acad. Sci. USA 89, 444–448 (1992).
Johnson, G., Tsao, P. S. & Lefer, A. M. Crit. Care Med. 19, 244–252 (1991).
Park, K. M. et al. J. Biol. Chem. 278, 27256–27266 (2003).
Flögel, U., Decking, U. K. M., Gödecke, A. & Schrader, J. J. Mol. Cell. Cardiol. 31, 827–836 (1999).
Endres, M. et al. Proc. Natl Acad. Sci. USA 95, 8880–8885 (1998).
Erkens, R. et al. Oxidat. Med. Cell. Longev. 2018, 8309698 (2018).
Parkins, C. S., Holder, A. L., Dennis, M. F., Stratford, M. R. L. & Chaplin, D. J. Free Radicals Res. 28, 271–281 (1998).
Stincone, A. et al. Biol. Rev. Camb. Phil. Soc. 90, 927–963 (2015).
Xiao, W., Wang, R.-S., Handy, D. E. & Loscalzo, J. Antioxidants Redox Signal. 28, 251–272 (2018).
Anand, P. et al. Proc. Natl Acad. Sci. USA 111, 18572–18577 (2014).
Israelsen, W. J. & Vander Heiden, M. G. Semin. Cell Dev. Biol. 43, 43–51 (2015).
Almeida, A., Moncada, S. & Bolaños, J. P. Nature Cell Biol. 6, 45–51 (2004).

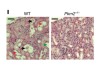 Read the paper: Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury
Read the paper: Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury
 Regeneration switch is a gas
Regeneration switch is a gas
 Nitric oxide caught in the traffic
Nitric oxide caught in the traffic