- NEWS AND VIEWS
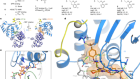
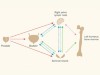
Leukaemia follows a blood-vessel track to enter the nervous system
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 560, 35-36 (2018)
doi: https://doi.org/10.1038/d41586-018-05693-x
References
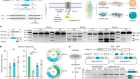
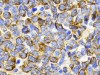
Yao, H. et al. Nature 560, 55–60 (2018).
Colognato, H., ffrench-Constant, C. & Feltri, M. L. Trends Neurosci. 28, 480–486 (2005).
Boire, A. et al. Cell 168, 1101–1113 (2017).
Kienast, Y. et al. Nature Med. 16, 116–122 (2010).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. PLoS ONE 4, e5857 (2009).
Thomas, L. B. Cancer Res. 25, 1555–1571 (1965).
Gritsenko, P. G., Ilina, O. & Friedl, P. J. Pathol. 226, 185–199 (2012).
Williams, M. T. S. et al. Blood 127, 1998–2006 (2016).
Osswald, M. et al. Nature 528, 93–98 (2015).
Münch, V. et al. Blood 130, 643–654 (2017).

 Read the paper: Leukaemia hijacks a neural mechanism to invade the central nervous system
Read the paper: Leukaemia hijacks a neural mechanism to invade the central nervous system
 Transition states that allow cancer to spread
Transition states that allow cancer to spread
 The complex seeds of metastasis
The complex seeds of metastasis