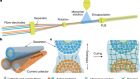
- NEWS AND VIEWS
Perfect union of protein and gel creates hyperexpandable crystals
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 557, 38-39 (2018)
doi: https://doi.org/10.1038/d41586-018-04956-x
References
Zhang, L., Bailey, J. B., Subramanian, R. H. & Tezcan, F. Nature 557, 86–91 (2018).
Lawson, D. M. et al. Nature 349, 541–544 (1991).
Roy, N., Bruchmann, B. & Lehn, J.-M. Chem. Soc. Rev. 44, 3786–3807 (2015).
Rother, M., Nussbaumer, M. G., Renggli, K. & Bruns, N. Chem. Soc. Rev. 45, 6213–6249 (2016).
Mann, S. Nature Mater. 8, 781–792 (2009).
Bale, J. B. et al. Science 353, 389–394 (2016).
Hsia, Y. et al. Nature 535, 136–139 (2016).

 Read the paper: Hyperexpandable, self-healing macromolecular crystals with integrated polymer networks
Read the paper: Hyperexpandable, self-healing macromolecular crystals with integrated polymer networks
 Lessons from tooth enamel
Lessons from tooth enamel
 Wood made denser and stronger
Wood made denser and stronger