- NEWS AND VIEWS
Molecular machines swap rings
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 557, 39-40 (2018)
doi: https://doi.org/10.1038/d41586-018-02732-5
References
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Chem. Rev. 115, 10081–10206 (2015).
Bissell, R. A., Córdova, E., Kaifer, A. E & Stoddart, J. F. Nature 369, 133–137 (1994).
Serreli, V., Lee, C.-F., Kay, E. R. & Leigh, D. A. Nature 445, 523–527 (2007).
Cheng, C. et al. Nature Nanotechnol. 10, 547–553 (2015).
Lewandowski, B. et al. Science 339, 189–193 (2013).
Lewis, J. E. M., Winn, J., Cera, L. & Goldup, S. M. J. Am. Chem. Soc. 138, 16329–16336 (2016).
Zhu, K., Baggi, G. & Loeb, S. J. Nature Chem. https://doi.org/10.1038/s41557-018-0040-9 (2018).
Zhu, K., O’Keefe, C. A., Vukotic, V. N., Schurko, R. W. & Loeb, S. J. Nature Chem. 7, 514–519 (2015).

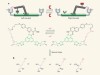 A molecular assembler
A molecular assembler
 No turning back for motorized molecules
No turning back for motorized molecules