- NEWS AND VIEWS
Kiss-and-tell way to track cell contacts
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 553, 414-415 (2018)
doi: https://doi.org/10.1038/d41586-018-00488-6
References
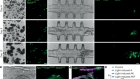
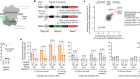
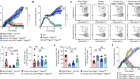
Pasqual, G. et al. Nature 553, 496–500 (2018).
Liu, D. S., Loh, K. H., Lam, S. S., White, K. A. & Ting, A. Y. PLoS ONE 8, e52823 (2013).
Mellman, I., Coukos, G. & Dranoff, G. Nature 480, 480–489 (2011).

 Read the paper: Monitoring T cell–dendritic cell interactions in vivo by intercellular enzymatic labelling
Read the paper: Monitoring T cell–dendritic cell interactions in vivo by intercellular enzymatic labelling
 Ultrasound approach tracks gut microbes
Ultrasound approach tracks gut microbes