Abstract
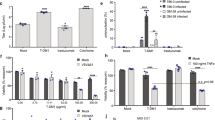
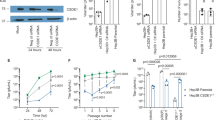
Oncolytic vesicular stomatitis virus (VSV) has potent antitumor activity but some cancer cells are resistant to VSV killing, either constitutively or due to type I interferon (IFN) inducing an antiviral state in the cells. Here, we evaluated VSV oncolysis of a panel of human head and neck cancer cells and showed that VSV resistance in SCC25 and SCC15 cells could be reversed with Janus kinase (JAK) 1/2 inhibitors (JAK inhibitor I and ruxolitinib). Pre-treatment of cells with JAK1/2 inhibitors before or in conjunction with VSV enhanced viral infection, spread and progeny yield (100- to 1000-fold increase). In contrast, inhibitors of histone deacetylase (LBH589), phosphatidylinositol 3-kinase (GDC-0941, LY294002), mammalian target of rapamycin (rapamycin) or signal transducer and activator of transcription 3 (STAT3 inhibitor VII) were ineffective. Compared with VSV-sensitive SW579 cells, IFNα/β responsive antiviral genes (IRF-9, IRF-7, OAS1 but not MxA) are constitutively expressed in SCC25 cells. Pretreatment with JAK inhibitors reduced mRNA levels of these genes, increasing VSV expression in the cells. Interestingly, 1 h of drug exposure was sufficient to reverse SCC25 resistance to VSV and was still effective if virus was added 24 h later. Overall, we show here that JAK inhibitor I and ruxolitinib (Jakafi) can reverse resistance to VSV, supporting the rationale to incorporate JAK1/2 inhibitors in future VSV virotherapy trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M . LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA 2013; 110: 7306–7311.
Russell SJ, Peng KW, Bell JC . Oncolytic virotherapy. Nat Biotechnol 2012; 30: 658–670.
Hastie E, Grdzelishvili VZ . Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol 2012; 93 (Pt 12): 2529–2545.
Reis JL Jr, Rodriguez LL, Mead DG, Smoliga G, Brown CC . Lesion development and replication kinetics during early infection in cattle inoculated with Vesicular stomatitis New Jersey virus via scarification and black fly (Simulium vittatum) bite. Vet Pathol 2011; 48: 547–557.
Rodriguez LL . Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res 2002; 85: 211–219.
Roberts A, Buonocore L, Price R, Forman J, Rose JK . Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol 1999; 73: 3723–3732.
Bi Z, Barna M, Komatsu T, Reiss CS . Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol 1995; 69: 6466–6472.
Clarke DK, Cooper D, Egan MA, Hendry RM, Parks CL, Udem SA . Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin Immunopathol 2006; 28: 239–253.
Publicover J, Ramsburg E, Rose JK . Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol 2004; 78: 9317–9324.
Kelly EJ, Nace R, Barber GN, Russell SJ . Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol 2010; 84: 1550–1562.
Ammayappan A, Nace R, Peng KW, Russell SJ . Neuroattenuation of vesicular stomatitis virus through picornaviral internal ribosome entry sites. J Virol 2013; 87: 3217–3228.
Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003; 4: 263–275.
Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS . Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol 2003; 77: 4646–4657.
Obuchi M, Fernandez M, Barber GN . Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 2003; 77: 8843–8856.
Jenks N, Myers R, Greiner SM, Thompson J, Mader EK, Greenslade A et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum Gene Ther 2010; 21: 451–462.
Naik S, Nace R, Barber GN, Russell SJ . Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-beta. Cancer Gene Ther 2012; 19: 443–450.
Naik S, Russell SJ . Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther 2009; 9: 1163–1176.
Heiber JF, Barber GN . Evaluation of innate immune signaling pathways in transformed cells. Methods Mol Biol 2012; 797: 217–238.
Sadler AJ, Williams BR . Interferon-inducible antiviral effectors. Nat Rev Immunol 2008; 8: 559–568.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD . How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264.
van Boxel-Dezaire AH, Rani MR, Stark GR . Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 2006; 25: 361–372.
de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 2001; 69: 912–920.
Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA 2008; 105: 14981–14986.
Carey BL, Ahmed M, Puckett S, Lyles DS . Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol 2008; 82: 12104–12115.
Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci USA 2010; 107: 1576–1581.
Hadac EM, Peng KW, Nakamura T, Russell SJ . Reengineering paramyxovirus tropism. Virology 2004; 329: 217–225.
Di Bona D, Cippitelli M, Fionda C, Camma C, Licata A, Santoni A et al. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol 2006; 45: 271–279.
Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol 2003; 74: 1125–1138.
Kawakubo K, Ohyashiki K, Ohyashiki JH, Shimamoto T, Fujimura T, Iwama H et al. A possible correlation between interferon-stimulated gene expression and cytogenetic responses in chronic myelogenous leukemia patients treated with alpha-interferon. Jpn J Clin Oncol 1996; 26: 59–64.
Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM . Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med 2006; 203: 41–46.
Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res 2006; 12 (20 Pt 1): 6170–6178.
Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett 2002; 12: 1219–1223.
Mascarenhas J, Mughal TI, Verstovsek S . Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib. Curr Med Chem 2012; 19: 4399–4413.
Yang CH, Murti A, Pfeffer LM . STAT3 complements defects in an interferon-resistant cell line: evidence for an essential role for STAT3 in interferon signaling and biological activities. Proc Natl Acad Sci USA 1998; 95: 5568–5572.
Chang HM, Paulson M, Holko M, Rice CM, Williams BR, Marie I et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci USA 2004; 101: 9578–9583.
Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol 2008; 9: 1157–1164.
Paglino JC, van den Pol AN . Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J Virol 2011; 85: 9346–9358.
Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ . Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology 2013; 436: 221–234.
Kontzias A, Kotlyar A, Laurence A, Changelian P, O'Shea JJ . Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr Opin Pharmacol 2012; 12: 464–470.
Vaddi K, Sarlis NJ, Gupta V . Ruxolitinib an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother 2012; 13: 2397–2407.
Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012; 18: 408–416.
Lane AA, Chabner BA . Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 2009; 27: 5459–5468.
Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 2008; 51: 5522–5532.
Fransson S, Uv A, Eriksson H, Andersson MK, Wettergren Y, Bergo M et al. p37delta is a new isoform of PI3K p110delta that increases cell proliferation and is overexpressed in tumors. Oncogene 2012; 31: 3277–3286.
Young NR, Liu J, Pierce C, Wei TF, Grushko T, Olopade OI et al. Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition. Mol Oncol 2013; 7: 359–368.
Uehara Y, Mochizuki M, Matsuno K, Haino T, Asai A . Novel high-throughput screening system for identifying STAT3-SH2 antagonists. Biochem Biophys Res Commun 2009; 380: 627–631.
Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994; 369: 756–758.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Geyer HL, Tibes R, Mesa RA . JAK2 inhibitors and their impact in myeloproliferative neoplasms. Hematology 2012; 17 (Suppl 1): S129–S132.
Mascarenhas J, Hoffman R . Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 2012; 18: 3008–3014.
Ahmed M, Marino TR, Puckett S, Kock ND, Lyles DS . Immune response in the absence of neurovirulence in mice infected with m protein mutant vesicular stomatitis virus. J Virol 2008; 82: 9273–9277.
Acknowledgements
We are grateful to funding support from Mayo Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SJR and KWP have financial interests in Omnis Pharma, an oncolytic VSV company.
Rights and permissions
About this article
Cite this article
Escobar-Zarate, D., Liu, YP., Suksanpaisan, L. et al. Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors. Cancer Gene Ther 20, 582–589 (2013). https://doi.org/10.1038/cgt.2013.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.55
Keywords
This article is cited by
-
Direct and indirect effects of IFN-α2b in malignancy treatment: not only an archer but also an arrow
Biomarker Research (2022)
-
JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models
Cancer Gene Therapy (2019)
-
Recombinant mumps virus as a cancer therapeutic agent
Molecular Therapy - Oncolytics (2016)
-
Application of interferon modulators to overcome partial resistance of human ovarian cancers to VSV-GP oncolytic viral therapy
Molecular Therapy - Oncolytics (2016)