Abstract
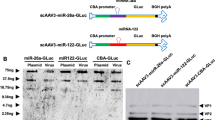
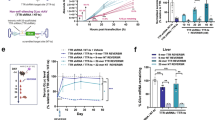
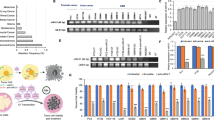
Adenoviral technology has been thoroughly evaluated for delivering genetic material to tumor tissue and the surrounding microenvironment. Almost any gene can be cloned into an adenovirus (Ad) vector, which when combined with strong, constitutively active promoters permit up to a million-fold amplification of the transgene in a single adenoviral particle, thus facilitating their use in cancer therapy and imaging. However, widespread infection of the liver and other non-targeted tissues by Ad vectors is a substantial problem that often results in significant liver inflammation and hepatotoxicity at doses required to achieve efficient tumor transduction. miR-122 is a highly expressed liver-specific microRNA (miRNA) that provides a unique opportunity for downregulating adenoviral transgene expression in liver tissue. The binding of endogenous miRNAs to complementary miRNA-targeting elements (miRTs) incorporated into the 3′ untranslated region of adenoviral transgenes interferes with message stability and/or protein translation, and miRT elements against miR-122 (miRT-122) can selectively reduce adenoviral transgene expression in the liver. Previous studies using miR-122-based regulation, with and without other types of transcriptional targeting, have yielded promising preliminary results. However, investigations to date evaluating miRT-122 elements for improving tumor specificity have used either non-tumor-bearing animals or direct intratumoral injection as the mode of delivery. In the present study, we confirmed the ability of miRT-122 sequences to selectively downregulate adenoviral luciferase expression in the liver in vitro and in vivo, and show that this strategy can improve tumor-specific transgene expression in a HT1080 human fibrosarcoma model. Rapid growth and the inefficient flow of blood through tumor neovasculature often results in profound hypoxia, which provides additional opportunities for targeting solid tumors and their microenvironment using vectors incorporating hypoxia-responsive promoters to drive transgene expression. We therefore used a combinatorial approach using miRT-122 elements with hypoxia-responsive transcriptional targeting to further improve the tumor-specific expression of an adenoviral reporter gene. Results from this investigation reveal that miRT122 elements alone decrease off-target liver expression and improve tumor specificity of adenoviral vectors. Furthermore, increased tumor specificity can be achieved by combining miRT-122 elements with hypoxia-responsive promoters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
www.wiley.co.uk/genmed/clinical [Web Page], J Gene Med Gene Therapy Clinical Trials Worldwide, Accessed on 23 October 2010.
Alemany R . Designing adenoviral vectors for tumor-specific targeting. Methods Mol Biol 2009; 542: 57–74.
Gerard RD, Chan L . Adenovirus-mediated gene transfer: strategies and applications in lipoprotein research. Curr Opin Lipidol 1996; 7: 105–111.
Gerard RD, Collen D . Adenovirus gene therapy for hypercholesterolemia, thrombosis and restenosis. Cardiovasc Res 1997; 35: 451–458.
Hogg RT, Garcia JA, Gerard RD . Adenoviral targeting of gene expression to tumors. Cancer Gene Ther 2010; 17: 375–386.
Hogg RT, Thorpe P, Gerard RD . Retargeting adenoviral vectors to improve gene transfer into tumors. Cancer Gene Ther 2011; 18: 275–287.
Praus M, Wauterickx K, Collen D, Gerard RD . Reduction of tumor cell migration and metastasis by adenoviral gene transfer of plasminogen activator inhibitors. Gene Ther 1999; 6: 227–236.
Varenne O, Sinnaeve P, Gillijns H, Iung B, Laurysens V, Meurrens K et al. Percutaneous gene therapy using recombinant adenoviruses encoding human herpes simplex virus thymidine kinase, human PAI-1, and human NOS3 in balloon-injured porcine coronary arteries. Hum Gene Ther 2000; 11: 1329–1339.
Liu Q, Muruve DA . Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther 2003; 10: 935–940.
Muruve DA, Barnes MJ, Stillman IE, Libermann TA . Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther 1999; 10: 965–976.
Worgall S, Wolff G, Falck-Pedersen E, Crystal RG . Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther 1997; 8: 37–44.
Lee RC, Feinbaum RL, Ambros V . The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854.
Croce CM . Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009; 10: 704–714.
Fabian MR, Sonenberg N, Filipowicz W . Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79: 351–379.
Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 2004; 1: 106–113.
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T . Identification of tissue-specific microRNAs from mouse. Curr Biol 2002; 12: 735–739.
Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A . miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol 2008; 48: 648–656.
Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K . Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol 2008; 82: 11009–11015.
Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW . Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog 2009; 5: e1000440.
Suzuki T, Sakurai F, Nakamura S, Kouyama E, Kawabata K, Kondoh M et al. miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy. Mol Ther 2008; 16: 1719–1726.
Wu C, Lin J, Hong M, Choudhury Y, Balani P, Leung D et al. Combinatorial control of suicide gene expression by tissue-specific promoter and microRNA regulation for cancer therapy. Mol Ther 2009; 17: 2058–2066.
Leja J, Nilsson B, Yu D, Gustafson E, Akerstrom G, Oberg K et al. Double-detargeted oncolytic adenovirus shows replication arrest in liver cells and retains neuroendocrine cell killing ability. PLoS One 2010; 5: e8916.
Neri D, Bicknell R . Tumour vascular targeting. Nat Rev Cancer 2005; 5: 436–446.
Tian H, McKnight SL, Russell DW . Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997; 11: 72–82.
McKnight SL, Gavis ER, Kingsbury R, Axel R . Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell 1981; 25: 385–398.
Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA . HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci 2008; 49: 2714–2720.
Aoki K, Barker C, Danthinne X, Imperiale MJ, Nabel GJ . Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med 1999; 5: 224–231.
Chang J, Guo JT, Jiang D, Guo H, Taylor JM, Block TM . Liver-specific microRNA miR-122 enhances the replication of hepatitis C virus in nonhepatic cells. J Virol 2008; 82: 8215–8223.
Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006; 99: 671–678.
Zaiss AK, Machado HB, Herschman HR . The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem 2009; 108: 778–790.
Cawood R, Wong SL, Di Y, Baban DF, Seymour LW . MicroRNA controlled adenovirus mediates anti-cancer efficacy without affecting endogenous microRNA activity. PLoS One 2011; 6: e16152.
DeMeritt IB, Milford LE, Yurochko AD . Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol 2004; 78: 4498–4507.
Hayden MS, West AP, Ghosh S . NF-kappaB and the immune response. Oncogene 2006; 25: 6758–6780.
Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 2001; 3: 28–35.
Acknowledgements
We thank TingTing Li for the kind gift of primary mouse hepatocytes. Alex Pertsemlidis provided statistical advice, and Joe Garcia and Rolf Brekken were kind enough to critically read the manuscript. This work was supported in part by NIH-NCI grant CA115935 to RDG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Card, P., Hogg, R., Gil del Alcazar, C. et al. MicroRNA silencing improves the tumor specificity of adenoviral transgene expression. Cancer Gene Ther 19, 451–459 (2012). https://doi.org/10.1038/cgt.2012.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.16
Keywords
This article is cited by
-
The application of oncolytic viruses in cancer therapy
Biotechnology Letters (2021)
-
Synergistic and independent action of endogenous microRNAs 122a and 199a for post-transcriptional liver detargeting of gene vectors
Scientific Reports (2018)
-
MicroRNA-regulated non-viral vectors with improved tumor specificity in an orthotopic rat model of hepatocellular carcinoma
Gene Therapy (2013)