Abstract
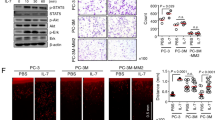
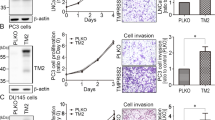
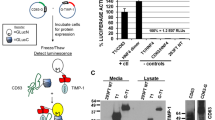
The destruction of extracellular matrix by matrix metalloproteinases is a key event in cancer progression. The tissue inhibitors of metalloproteinases can restrain tumor growth by inhibiting these enzymes. We sought to determine whether overexpression of tissue inhibitor of metalloproteinase-3 (TIMP-3) could suppress the malignant phenotype of human prostate cancer cell line PC-3M. Stable overexpression of TIMP-3 inhibited cell proliferation significantly by MTT assay. Both early and late apoptosis were observed in TIMP-3 overexpressing cells, and flow cytometry analysis showed S-phase blocking of the cell cycle. Monolayer invasion assay and transwell invasion assay showed significantly decreased invasive potential in TIMP-3 overexpressing cells compared with control cells. Cell adhesion and motility were also lower after TIMP-3 was overexpressed. In vivo, cells stably overexpressing TIMP-3 completely lost the ability to form tumors after injection into nude mice. Transfection of TIMP-3 into established tumors by electroporation also had a significant antitumor effect. TIMP-3-treated tumor tissues had significant apoptosis by TUNEL assay. These results showed that overexpression of TIMP-3 inhibits invasion and proliferation of prostate cancer cells in vitro and inhibits tumor growth in vivo. The experiments suggest a potential use for TIMP-3 in the gene therapy of prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Handsley MM, Edwards DR . Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer 2005; 115: 849–860.
Murray GI . Matrix metalloproteinases: a multifunctional group of molecules. J Pathol 2001; 195: 135–137.
Tu G, Xu W, Huang H, Li S . Progress in the development of matrix metalloproteinase inhibitors. Curr Med Chem 2008; 15: 1388–1395.
Lee MH, Atkinson S, Murphy G . Identification of the extracellular matrix (ECM) binding motifs of tissue inhibitor of metalloproteinases (TIMP)-3 and effective transfer to TIMP-1. J Biol Chem 2007; 282: 6887–6898.
Cruz-Munoz W, Kim I, Khokha R . TIMP-3 deficiency in the host, but not in the tumor, enhances tumor growth and angiogenesis. Oncogene 2006; 25: 650–655.
Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE et al. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene 2003; 22: 2121–2134.
Tran PL, Vigneron JP, Pericat D, Dubois S, Cazals D, Hervy M et al. Gene therapy for hepatocellular carcinoma using non-viral vectors composed of bis guanidinium-tren-cholesterol and plasmids encoding the tissue inhibitors of metalloproteinases TIMP-2 and TIMP-3. Cancer Gene Ther 2003; 10: 435–444.
Edwards DR . TIMP-3 and endocrine therapy of breast cancer: an apoptosis connection emerges. J Pathol 2004; 202: 391–394.
Finan KM, Hodge G, Reynolds AM, Hodge S, Holmes MD, Baker AH et al. In vitro susceptibility to the pro-apoptotic effects of TIMP-3 gene delivery translates to greater in vivo efficacy versus gene delivery for TIMPs-1 or -2. Lung Cancer 2006; 53: 273–284.
Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM . Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood 2002; 100: 3361–3368.
Karan D, Lin FC, Bryan M, Ringel J, Moniaux N, Lin MF et al. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol 2003; 23: 1365–1371.
Connolly JM, Rose DP . Angiogenesis in two human prostate cancer cell lines with differing metastatic potential when growing as solid tumors in nude mice. J Urol 1998; 160 (3 Pt 1): 932–936.
Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao D et al. Down-regulation of signal transducer and activator of transcription 3 expression using vector-based small interfering RNAs suppresses growth of human prostate tumor in vivo. Clin Cancer Res 2005; 11: 6333–6341.
Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, Hill RS et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet 2004; 36: 69–76.
Zhang L, Gao L, Li Y, Lin G, Shao Y, Ji K et al. Effects of plasmid-based Stat3-specific short hairpin RNA and GRIM-19 on PC-3M tumor cell growth. Clin Cancer Res 2008; 14: 559–568.
Cheresh DA, Stupack DG . Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 2008; 27: 6285–6298.
Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res 2008; 68: 1170–1179.
Chirco R, Liu XW, Jung KK, Kim HR . Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 2006; 25: 99–113.
Jiang Y, Goldberg ID, Shi YE . Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 2002; 21: 2245–2252.
Stetler-Stevenson WG . Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 2008; 1: re6.
Spurbeck WW, Ng CY, Vanin EF, Davidoff AM . Retroviral vector-producer cell-mediated in vivo gene transfer of TIMP-3 restricts angiogenesis and neuroblastoma growth in mice. Cancer Gene Ther 2003; 10: 161–167.
Han X, Zhang H, Jia M, Han G, Jiang W . Expression of TIMP-3 gene by construction of a eukaryotic cell expression vector and its role in reduction of metastasis in a human breast cancer cell line. Cell Mol Immunol 2004; 1: 308–310.
Gao LF, Xu DQ, Wen LJ, Zhang XY, Shao YT, Zhao XJ . Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells. Acta Pharmacol Sin 2005; 26: 377–383.
Bond M, Murphy G, Bennett MR, Newby AC, Baker AH . Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol Chem 2002; 277: 13787–13795.
Bian J, Wang Y, Smith MR, Kim H, Jacobs C, Jackman J et al. Suppression of in vivo tumor growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis 1996; 17: 1805–1811.
Yang TT, Hawkes SP . Role of the 21-kDa protein TIMP-3 in oncogenic transformation of cultured chicken embryo fibroblasts. Proc Natl Acad Sci USA 1992; 89: 10676–10680.
Zhang Y, Qian H, Lin C, Lang J, Xiang Y, Fu M et al. Adenovirus carrying TIMP-3: a potential tool for cervical cancer treatment. Gynecol Oncol 2008; 108: 234–240.
Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC . Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer 1999; 79: 1347–1355.
Wild A, Ramaswamy A, Langer P, Celik I, Fendrich V, Chaloupka B et al. Frequent methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene in pancreatic endocrine tumors. J Clin Endocrinol Metab 2003; 88: 1367–1373.
Sun Y, Kim H, Parker M, Stetler-Stevenson WG, Colburn NH . Lack of suppression of tumor cell phenotype by overexpression of TIMP-3 in mouse JB6 tumor cells identification of a transfectant with increased tumorigenicity and invasiveness. Anticancer Res 1996; 16: 1–7.
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30.
Zhang L, Wu S, Guo LR, Zhao XJ . Diagnostic strategies and the incidence of prostate cancer: reasons for the low reported incidence of prostate cancer in China. Asian J Androl 2009; 11: 9–13.
Zhang L, Ji G, Li X, Li S, Gao H, Gan H et al. The influence of mass screening for prostate cancer on the diagnostic status of the clinical prostate cancer. Chin J Urol 2004; 25: 103–104.
Mao HL, Zhu ZQ, Chen CD . The androgen receptor in hormone-refractory prostate cancer. Asian J Androl 2009; 11: 69–73.
Shirakawa T, Fujisawa M, Gotoh A . Gene therapy in prostate cancer: past, present and future. Front Biosci 2008; 13: 2115–2119.
Alexandrova AY . Evolution of cell interactions with extracellular matrix during carcinogenesis. Biochemistry (Mosc) 2008; 73: 733–741.
Sanlioglu AD, Karacay B, Koksal IT, Griffith TS, Sanlioglu S . DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther 2007; 14: 976–984.
Deng X, He G, Levine A, Cao Y, Mullins C . Adenovirus-mediated expression of TIMP-1 and TIMP-2 in bone inhibits osteolytic degradation by human prostate cancer. Int J Cancer 2008; 122: 209–218.
Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther 2007; 15: 834–840.
Horiguchi A, Zheng R, Goodman Jr OB, Shen R, Guan H, Hersh LB et al. Lentiviral vector neutral endopeptidase gene transfer suppresses prostate cancer tumor growth. Cancer Gene Ther 2007; 14: 583–589.
Amalfitano A, Parks RJ . Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther 2002; 2: 111–133.
Gothelf A, Mir LM, Gehl J . Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev 2003; 29: 371–387.
Gao LF, Wen LJ, Yu H, Zhang L, Meng Y, Shao YT et al. Knockdown of Stat3 expression using RNAi inhibits growth of laryngeal tumors in vivo. Acta Pharmacol Sin 2006; 27: 347–352.
Lam P, Sian Lim K, Mei Wang S, Hui KM . A microarray study to characterize the molecular mechanism of TIMP-3-mediated tumor rejection. Mol Ther 2005; 12: 144–152.
Smith MR, Kung H, Durum SK, Colburn NH, Sun Y . TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine 1997; 9: 770–780.
Zhang L, Gao L, Zhao L, Guo B, Ji K, Tian Y et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res 2007; 67: 5859–5864.
Acknowledgements
This work was funded by National Natural Science Foundation of China (No. 30801354) and Jilin Provincial Science & Technology Department (No. 20080154). We thank Professor Frederick William Orr for his critical reading and revising our paper.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, L., Zhao, L., Zhao, D. et al. Inhibition of tumor growth and induction of apoptosis in prostate cancer cell lines by overexpression of tissue inhibitor of matrix metalloproteinase-3. Cancer Gene Ther 17, 171–179 (2010). https://doi.org/10.1038/cgt.2009.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.59
Keywords
This article is cited by
-
Loss of TIMP3 by promoter methylation of Sp1 binding site promotes oral cancer metastasis
Cell Death & Disease (2019)
-
Plasmid-based Survivin shRNA and GRIM-19 carried by attenuated Salmonella suppresses tumor cell growth
Asian Journal of Andrology (2012)
-
Plasmid-based Stat3 siRNA delivered by hydroxyapatite nanoparticles suppresses mouse prostate tumour growth in vivo
Asian Journal of Andrology (2011)