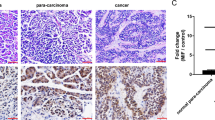
Abstract
A panel of kinases whose inhibition increased apoptosis of pancreatic adenocarcinoma cells in vitro was recently established. The aim of this work was to observe in a mouse xenograft model whether inhibition of these kinases would alter pancreatic tumor growth. Rate of apoptosis, caspase-3 activity and cell viability were assessed in two pancreatic cancer cell lines, MiaPaCa2 and BxPC3, after inhibiting selected kinases by transfection of specific siRNAs. For in vivo experiments, MiaPaCa2 cells were injected into the pancreas of nude mice, where they formed tumors. Inhibition of kinases was obtained by repeated intraperitoneal (i.p.) injections of modified O-Methyl (OMe) siRNAs specific for the selected kinases. Tumor volumes were assessed after 21 days. Among selected kinases, PAK7, MAP3K7 and CK2α were those whose inhibition increased apoptosis the most in vitro. Simultaneous inhibition of two of them increased apoptosis up to five times. Moreover, inhibiting these kinases had little effect on 10 non-pancreatic cell lines, suggesting pancreatic specificity. In vivo, OMe-siRNAs induced significant but incomplete inhibition of kinase expression (45–75%). Nevertheless, such inhibition resulted in a twofold increase in caspase-3 activity in tumors and a strong reduction in tumor volume (about 75%). In vivo inhibition by OMe-siRNAs of three survival kinases apparently specific for pancreatic cancer cells, PAK7, MAP3K7 and CK2α, decreases significantly the growth of xenografted MiaPaCa2 cells. This strategy is therefore of potential clinical interest.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E et al. Cancer statistics, 2004 CA. Cancer J Clin 2004; 54: 8–29.
Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413.
Richards DA . Chemotherapeutic gemcitabine doublets in pancreatic carcinoma. Semin Oncol 2005; 32: S9–S13.
Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 2006; 94: 481–485.
Friberg G, Kindler HL . Chemotherapy for advanced pancreatic cancer: past, present, and future. Curr Oncol Rep 2005; 7: 186–195.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S . The protein kinase complement of the human genome. Science 2002; 298: 1912–1934.
Giroux V, Iovanna J, Dagorn JC . Probing the human kinome for kinases involved in pancreatic cancer cell survival and gemcitabine resistance. Faseb J 2006; 20: 1982–1991.
Carracedo A, Gironella M, Lorente M, Garcia S, Guzmán M, Velasco G et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 2006; 66: 6748–6755.
Judge AD, Bola G, Lee AC, MacLachlan I . Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo . Mol Ther 2006; 13: 494–505.
Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Höbel S et al. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther 2006; 17: 751–766.
Soverini S, Martinelli G, Iacobucci I, Baccarani M . Imatinib mesylate for the treatment of chronic myeloid leukemia. Expert Rev Anticancer Ther 2008; 8: 853–864.
Cabebe E, Wakelee H . Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol 2007; 8: 15–27.
Tuveson DA, Zhu L, Gopinathan A, Willis NA, Kachatrian L, Grochow R et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res 2006; 66: 242–247.
Cotteret S, Jaffer ZM, Beeser A, Chernoff J . p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol 2003; 23: 5526–5539.
Cotteret S, Chernoff J . Nucleocytoplasmic shuttling of Pak5 regulates its antiapoptotic properties. Mol Cell Biol 2006; 26: 3215–3230.
Roberts AB, Sporn MB . Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth factors 1993; 8: 1–9.
Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995; 270: 2008–2011.
Sakurai H, Shigemori N, Hasegawa K, Sugita T . TGF-beta-activated kinase 1 stimulates NF-kappa B activation by an NF-kappa B-inducing kinase-independent mechanism. Biochem Biophys Res Commun 1998; 243: 545–549.
Sakurai H, Miyoshi H, Toriumi W, Sugita T . Functional interactions of transforming growth factor beta-activated kinase 1 with IkappaB kinases to stimulate NF-kappaB activation. J Biol Chem 1999; 274: 10641–10648.
Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene 2003; 22: 412–425.
Sanna MG, da Silva Correia J, Ducrey O, Lee J, Nomoto K, Schrantz N et al. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol 2002; 22: 1754–1766.
Guo C, Yu S, Davis AT, Wang H, Green JE, Ahmed K . A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem 2001; 276: 5992–5999.
Ahmed K, Gerber DA, Cochet C . Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 2005; 12: 226–230.
Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K . Protein kinase CK2 signal in neoplasia. Histol Histopathol 2001; 16: 573–582.
Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell 2001; 8: 601–611.
Li PF, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R . Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol Cell 2002; 10: 247–258.
Faust RA, Tawfic S, Davis AT, Bubash LA, Ahmed K . Antisense oligonucleotides against protein kinase CK2-alpha inhibit growth of squamous cell carcinoma of the head and neck in vitro . Head Neck 2000; 22: 341–346.
Ravi R, Bedi A . Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res 2002; 62: 4180–4185.
Ruzzene M, Penzo D, Pinna LA . Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J 2002; 364: 41–47.
Hamacher R, Saur D, Fritsch R, Reichert M, Schmid RM, Schneider G . Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol Rep 2007; 18: 695–701.
Acknowledgements
This work was supported by grants from the INSERM, the Canceropole PACA and the Association pour la Recherche sur le Cancer (# 3932). JLI and JCD are supported by a ‘Contrat Interface’ INSERM–AP–HM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giroux, V., Iovanna, J., Garcia, S. et al. Combined inhibition of PAK7, MAP3K7 and CK2α kinases inhibits the growth of MiaPaCa2 pancreatic cancer cell xenografts. Cancer Gene Ther 16, 731–740 (2009). https://doi.org/10.1038/cgt.2009.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.22
Keywords
This article is cited by
-
PAK5-mediated phosphorylation and nuclear translocation of NF-κB-p65 promotes breast cancer cell proliferation in vitro and in vivo
Journal of Experimental & Clinical Cancer Research (2017)
-
P21-activated kinase 5 plays essential roles in the proliferation and tumorigenicity of human hepatocellular carcinoma
Acta Pharmacologica Sinica (2014)
-
The overexpression of P21-activated kinase 5 (PAK5) promotes paclitaxel-chemoresistance of epithelial ovarian cancer
Molecular and Cellular Biochemistry (2013)