Abstract
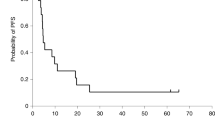
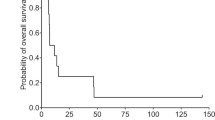
In this study, we investigated the safety and efficacy in cancer patients of a single intra-tumor injection of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene (AdV/TK) followed by systemic administration of ganciclovir (GCV). In 18 patients with malignant tumors refractory to standard treatment, AdV/TK was injected on day 1 with dose escalation from 2.5 × 1011 to 1 × 1012 virus particles (VP), and GCV (5 mg kg−1) was delivered intravenously every 12 h from days 2 to 15. The most common treatment-related toxicities were transient fever (10/18) and local injection site reaction (10/18), and most adverse events were WHO grade I/II. Anti-adenovirus antibody levels increased continuously during treatment, but anti-HSV antibody levels remained stable. One patient had a PR at the injection site but PD was found in the primary site (lung cancer), one patient with fibrosarcoma of the neck had an MR, five patients had SD, and 10 patients had PD. In conclusion, AdV/TK followed by GCV can be administered safely to Chinese cancer patients, and achieved a local response with few environmental effects. Because the response was localized, single regional tumor relapse, especially after radiation, may be an indication for this suicide gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Harvey BG, Maroni J, O’Donoghue KA, Chu KW, Muscat JC, Pippo AL et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther 2002; 13: 15–63.
Sung MW, Yeh HC, Thung SN, Schwartz ME, Mandeli JP, Chen SH et al. Intratumoral adenovirus-mediated suicide gene transfer for hepatic metastases from colorectal adenocarcinoma: results of a phase I clinical trial. Mol Ther 2001; 4: 182–191.
Harvey BG, Hackett NR, El-Sawy T, Rosengart TK, Hirschowitz EA, Lieberman MD et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol 1999; 73: 6729–6742.
Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL . Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo . Proc Natl Acad Sci USA 1994; 91: 3054–3057.
Thompson TC . In situ gene therapy for prostate cancer. Oncol Res 1999; 11: 1–8.
Hall SJ, Mutchnik SE, Chen SH, Woo SL, Thompson TC . Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer 1997; 70: 183–187.
Hall SJ, Sanford MA, Atkinson G, Chen SH . Induction of potent antitumor natural killer cell activity by herpes simplex virus-thymidine kinase and ganciclovir therapy in an orthotopic mouse model of prostate cancer. Cancer Res 1998; 58: 3221–3225.
Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther 2007; 15: 834–840.
Shirakawa T, Terao S, Hinata N, Tanaka K, Takenaka A, Hara I . et al. Long-term outcome of phase I/II clinical trial of Ad-OC-TK/VAL gene therapy for hormone-refractory metastatic prostate cancer. Hum Gene Ther 2007; 18: 1225–1232.
Li N, Zhou J, Weng D, Zhang C, Li L, Wang B et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2007; 13: 5847–5854.
Chévez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol 2005; 23: 7927–7935.
Sterman DH, Recio A, Vachani A, Sun J, Cheung L, DeLong P et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res 2005; 11: 7444–7453.
Alvarez RD, Gomez-Navarro J, Wang M, Barnes MN, Strong TV, Arani RB et al. Adenoviral-mediated suicide gene therapy for ovarian cancer. Mol Ther 2000; 2: 524–530.
Sterman DH, Treat J, Litzky LA, Amin KM, Coonrod L, Molnar-Kimber K et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther 1998; 9: 1083–1092.
Slos P, De Meyer M, Leroy P, Rousseau C, Acres B . Immunotherapy of established tumors in mice by intratumoral injection of an adenovirus vector harboring the human IL-2 cDNA: induction of CD8 (+) T-cell immunity and NK activity. Cancer Gene Ther 2001; 8: 321–332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, F., Li, S., Li, XL. et al. Phase I and biodistribution study of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene and ganciclovir administration in patients with head and neck cancer and other malignant tumors. Cancer Gene Ther 16, 723–730 (2009). https://doi.org/10.1038/cgt.2009.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2009.19
Keywords
This article is cited by
-
Strategies for delivering therapeutics across the blood–brain barrier
Nature Reviews Drug Discovery (2021)
-
Gene therapy research in Asia
Gene Therapy (2017)
-
Drug discovery in advanced prostate cancer: translating biology into therapy
Nature Reviews Drug Discovery (2016)
-
Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity
Cancer Gene Therapy (2014)
-
Chemovirotherapy for head and neck squamous cell carcinoma with EGFR-targeted and CD/UPRT-armed oncolytic measles virus
Cancer Gene Therapy (2012)