Abstract
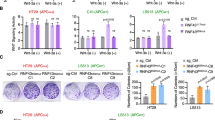
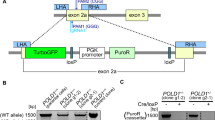
Cancer is one of the diseases for which RNA interference is a potential therapeutic approach. Genes involved in the promotion or maintenance of tumor growth are obvious targets for RNAi. RNAi is also considered an attractive additional approach to conventional chemotherapy for cancer treatment. Moreover, siRNAs have shown a high specificity for their molecular target mRNAs as they can selectively inhibit cancer-promoting genes that differ by a point mutation. Loss of heterozygosity (LOH) reduces genes to hemizygosity in cancer cells and presents an absolute difference between normal and cancer cells. The regions of LOH are usually much larger than the tumor suppressor gene, which is lost, and has been shown to contain genes that are essential for cell survival. Single-nucleotide polymorphisms (SNPs) are the most common type of genetic variation in man. SNPs in essential genes that are frequently affected by LOH can be used as a target for a therapy against cancer cells with LOH. We have designed siRNAs against the gene of the large subunit of RNA polymerase II (POLR2A), a gene located in close proximity to the tumor suppressor gene p53, which frequently shows LOH in cancer cells. It is shown in vitro that siRNA can selectively inhibit POLR2A expression dependent on its genotype. Furthermore, cancer cell proliferation and tumor growth inhibition in nude mice was genotype dependent. We conclude that siRNA can be used for genotype-specific inhibition of tumor growth targeting an SNP in POLR2A in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- ASI:
-
allele-specific inhibition
- DMEM:
-
Dulbecco's modified Eagle's medium
- eEF2α:
-
elongation factor-2α
- LOH:
-
loss of heterozygosity
- ODNs:
-
antisense oligonucleotides
- POLR2A:
-
large subunit of RNA polymerase II
- PVDF:
-
polyvinylidene fluoride
- RNAi:
-
RNA interference
- RPA70:
-
replication protein A, 70-kDa subunit
- SNPs:
-
single nucleotide polymorphisms
- SDS–PAGE:
-
SDS–polycrylamide gel electrophoresis
References
Leung RK, Whittaker PA . RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther 2005; 107: 222–239.
Brummelkamp TR, Bernards R, Agami R . Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002; 2: 243–247.
Zhang Z, Jiang G, Yang F, Wang J . Knockdown of mutant K-ras expression by adenovirus-mediated siRNA inhibits the in vitro and in vivo growth of lung cancer cells. Cancer Biol Ther 2006; 5: 1481–1486.
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806–811.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Kim DH, Rossi JJ . Strategies for silencing human disease using RNA interference. Nat Rev Genet 2007; 8: 173–184.
Aigner A . Applications of RNA interference: current state and prospects for siRNA-based strategies in vivo. Appl Microbiol Biotechnol 2007; 76: 9–21.
Lengauer C, Kinzler KW, Vogelstein B . Genetic instabilities in human cancers. Nature 1998; 396: 643–649.
Basilion JP, Schievella AR, Burns E, Rioux P, Olson JC, Monia BP et al. Selective killing of cancer cells based on loss of heterozygosity and normal variation in the human genome: a new paradigm for anticancer drug therapy. Mol Pharmacol 1999; 56: 359–369.
Fluiter K, Housman D, Ten Asbroek AL, Baas F . Killing cancer by targeting genes that cancer cells have lost: allele-specific inhibition, a novel approach to the treatment of genetic disorders. Cell Mol Life Sci 2003; 60: 834–843.
Ten Asbroek AL, Olsen J, Housman D, Baas F, Stanton Jr V . Genetic variation in mRNA coding sequences of highly conserved genes. Physiol Genomics 2001; 5: 113–118.
Fluiter K, ten Asbroek AL, van Groenigen M, Nooij M, Aalders MC, Baas F . Tumor genotype-specific growth inhibition in vivo by antisense oligonucleotides against a polymorphic site of the large subunit of human RNA polymerase II. Cancer Res 2002; 62: 2024–2028.
Abdelgany A, Wood M, Beeson D . Allele-specific silencing of a pathogenic mutant acetylcholine receptor subunit by RNA interference. Hum Mol Genet 2003; 12: 2637–2644.
Ding H, Schwarz DS, Keene A, Affar el B, Fenton L, Xia X et al. Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell 2003; 2: 209–217.
Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, Lozano G et al. Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci USA 2002; 99: 14849–14854.
Miller VM, Gouvion CM, Davidson BL, Paulson HL . Targeting Alzheimer's disease genes with RNA interference: an efficient strategy for silencing mutant alleles. Nucleic Acids Res 2004; 32: 661–668.
Miller VM, Xia H, Marrs GL, Gouvion CM, Lee G, Davidson BL et al. Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci USA 2003; 100: 7195–7200.
Du Q, Thonberg H, Wang J, Wahlestedt C, Liang Z . A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res 2005; 33: 1671–1677.
Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Burchard J et al. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet 2006; 2: 1307–1318.
ten Asbroek AL, Fluiter K, van Groenigen M, Nooij M, Baas F . Polymorphisms in the large subunit of human RNA polymerase II as target for allele-specific inhibition. Nucleic Acids Res 2000; 28: 1133–1138.
Church GM, Gilbert W . Genomic sequencing. Proc Natl Acad Sci USA 1984; 81: 1991–1995.
Duxbury MS, Matros E, Ito H, Zinner MJ, Ashley SW, Whang EE . Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer. Ann Surg 2004; 240: 667–674; discussion 675–676.
Mook OR, Baas F, de Wissel MB, Fluiter K . Evaluation of locked nucleic acid-modified small interfering RNA in vitro and in vivo. Mol Cancer Ther 2007; 6: 833–843.
Meyer T, Regenass U, Fabbro D, Alteri E, Rosel J, Muller M et al. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer 1989; 43: 851–856.
Kurosawa T, Igarashi S, Nishizawa M, Onodera O . Selective silencing of a mutant transthyretin allele by small interfering RNAs. Biochem Biophys Res Commun 2005; 337: 1012–1018.
Dykxhoorn DM, Schlehuber LD, London IM, Lieberman J . Determinants of specific RNA interference-mediated silencing of human beta-globin alleles differing by a single nucleotide polymorphism. Proc Natl Acad Sci USA 2006; 103: 5953–5958.
Li Y, Yokota T, Matsumura R, Taira K, Mizusawa H . Sequence-dependent and independent inhibition specific for mutant ataxin-3 by small interfering RNA. Ann Neurol 2004; 56: 124–129.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE . EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene 2004; 23: 1448–1456.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE . RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene 2004; 23: 1539–1548.
Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H . Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res 2005; 65: 967–971.
Ocker M, Neureiter D, Lueders M, Zopf S, Ganslmayer M, Hahn EG et al. Variants of bcl-2 specific siRNA for silencing antiapoptotic bcl-2 in pancreatic cancer. Gut 2005; 54: 1298–1308.
Acknowledgements
This work was supported by the Dutch Cancer Society, project number 2003-2968 and the Stichting Kindergeneeskundig Kanker Onderzoek.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mook, O., Baas, F., de Wissel, M. et al. Allele-specific cancer cell killing in vitro and in vivo targeting a single-nucleotide polymorphism in POLR2A. Cancer Gene Ther 16, 532–538 (2009). https://doi.org/10.1038/cgt.2008.104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2008.104
Keywords
This article is cited by
-
Silencing the FOP gene
Gene Therapy (2012)