Abstract
The rapid clearance of dying cells is important for the well-being of multicellular organisms. In C. elegans, cell corpse removal is mainly mediated by three parallel engulfment signaling cascades. These pathways include two small GTPases, MIG-2/RhoG and CED-10/Rac1. Here we present the identification and characterization of CDC-42 as a third GTPase involved in the regulation of cell corpse clearance. Genetic analyses performed by both loss of cdc-42 function and cdc-42 overexpression place cdc-42 in parallel to the ced-2/5/12 signaling module, in parallel to or upstream of the ced-10 module, and downstream of the ced-1/6/7 module. CDC-42 accumulates in engulfing cells at membranes surrounding apoptotic corpses. The formation of such halos depends on the integrins PAT-2/PAT-3, UNC-112 and the GEF protein UIG-1, but not on the canonical ced-1/6/7 or ced-2/5/12 signaling modules. Together, our results suggest that the small GTPase CDC-42 regulates apoptotic cell engulfment possibly upstream of the canonical Rac GTPase CED-10, by polarizing the engulfing cell toward the apoptotic corpse in response to integrin signaling and ced-1/6/7 signaling in C. elegans.
Similar content being viewed by others
Main
During development and in tissue homeostasis, multicellular organisms frequently use apoptosis to eliminate cells that are useless or potentially dangerous. Apoptotic cells are readily recognized, internalized and degraded by neighboring or specialized engulfing cells. Rapid clearance of unwanted cells avoids the release of harmful intracellular contents into the surroundings that can lead to inflammation and autoimmune disease.1
The nematode C. elegans serves as a simple yet powerful genetic model organism to study cell corpse clearance in vivo. Many genes involved in recognition, internalization or degradation of apoptotic corpses have been identified through forward and reverse genetic screens in the past two decades.2 Loss of engulfment activity not only results in the persistence of cell corpses, but also leads to the survival of some cells destined to die,3 and – in some cases – leads to impaired cell migration.4
Phenotypic, genetic and biochemical analyses of the early ‘classical’ ced (cell death abnormal) genes led to the identification of three partially redundant signaling cascades that cooperate to regulate cytoskeletal rearrangements and the migration of the engulfing cell around the apoptotic corpse.5, 6, 7, 8, 9 In the first pathway, the transmembrane protein CED-1/MEGF10 has been proposed to act as a cell corpse receptor10 that binds to exposed phosphatidylserine (PS), either directly or indirectly through the action of the bridging molecule TTR-52/TTR.11, 12 The lipid transporter homolog CED-7 also plays a role at this stage, at least in part by promoting the exposure of PS in the outer leaflet of the doomed cell.13 The adaptor protein CED-6/GULP transduces the signal(s) from CED-1 downstream to CED-10/Rac1 and DYN-1/Dynamin to drive cytoskeletal rearrangements and phagosome maturation.8, 14, 15, 16 In the second pathway, activation of CED-10 is promoted by the bipartite GEF (guanine exchange factor) complex composed of CED-12/Elmo–CED-5/Dock180.17, 18, 19, 20 This GEF complex in turn is regulated by CED-2/CrkII and the small GTPase MIG-2/RhoG. In the third pathway, the cytoskeletal regulator ABL-1/Abl suppresses corpse clearance by inhibiting ABI-1/Abl-interacting protein.21 Active GTP-loaded CED-10 promotes the extensive cytoskeletal rearrangements that are essential for proper cell corpse internalization.8 This process is negatively regulated by the GTPase-activating protein (GAP) SRGP-1/srGAP1 that facilitates GTP hydrolysis in CED-10.22
Here we present the identification and characterization of cdc-42 (cell division control protein-42) as an additional mediator of engulfment signaling regulated by SRGP-1 (Slit/Robo GTPase activating protein 1). Our epistatic analyses, performed with cdc-42(lf) mutants and cdc-42 overexpression experiments, suggest that cdc-42 acts downstream or in parallel to the ced-1/6/7 and in parallel to the ced-2/5/12 signaling cascades. Using a functional and rescuing GFP::CDC-42 protein, we show that CDC-42 is recruited to the cell membrane surrounding apoptotic corpses, and that this localization requires the integrin-α PAT-2 but not the canonical ced-1/6/7 or ced-2/5/12 cascades.
Taken together, our results suggest that the small GTPase CDC-42 regulates apoptotic cell engulfment upstream of the canonical Rac GTPase CED-10, possibly by polarizing the engulfing cell toward the apoptotic corpse in response to integrin signaling. Our data confirm and significantly expand on recent results published by Hsieh et al.,23 who independently identified CDC-42 as an engulfment regulator downstream of integrin-α PAT-2.
Results
Identification of cdc-42 as a mediator of engulfment signaling in C. elegans
We previously reported the identification of SRGP-1 as a negative regulator of corpse engulfment.22 Biochemical and genetic evidence suggested that SRGP-1 acts as a GAP for the Rac protein CED-10. Because loss of ced-10 only partially eliminated the suppressive activity of srgp-1 mutations, we surmised that srgp-1 might also modulate other Rho GTPases important for cell corpse clearance (Figure 1a).
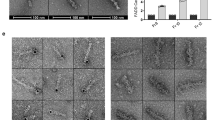
CDC-42 is a candidate SRGP-1 target during engulfment signaling. (a) Knockdown of cdc-42 and loss of srgp-1(ok300) have opposite effects on cell corpse clearance in C. elegans. Staged animals (P0) were grown on RNAi plates and freshly hatched L1 progeny larvae (F1) were scored for corpse numbers in the head region. All strains carry the ced-5(tm1949) mutation in the background. Data are shown as average±S.D. (n≥25). rho-1(RNAi) causes embryonic lethality and thus is not done (n.d.). (b) The SRGP-1 GAP domain binds GTP-bound CDC-42 in vitro. The GTP- and GDP-bound isoforms of 6xHis-tagged CDC-42 and RHO-1 (CDC-42: Q61L and T17N, RHO-1: Q63L and T19N, respectively) were used for pulldowns. GST-fusions used: GST (alone), the GAP domain of SRGP-1, the GTP hydrolysis-deficient SRGP-1 GAP (R563A) domain22 and the GAP domain of RGA-3, a potential GAP of both RHO-1 and CDC-42 (see Schonegg and Hyman26)
In order to identify these candidate GTPases, we used RNA interference (RNAi) to knock down known Rho family members (including rac-2, crp-1, chw-1, rho-1, cdc-42 and ced-10) in a sensitized ced-5; srgp-1 double mutant background. ced-5 mutants contain many persistent cell corpses in larval L1 heads as compared with wild type. These corpse numbers are significantly reduced in ced-5; srgp-1 double mutants, at least in part because of an increased activity of CED-10/Rac1 (Supplementary Figure S1).22 We found that in this sensitized background, reduction of cdc-42 function not only fully reverted the srgp-1 suppressor phenotype of ced-5 mutants, but also significantly enhanced the corpse persistent phenotype of ced-5 single mutants (Figure 1a). We therefore concluded that cdc-42 might be an additional candidate Rho GTPase involved in cell corpse clearance in C. elegans.
We previously showed that the GAP domain of SRGP-1 binds GTP-bound (active) CED-10/Rac1 in vitro.22 Using bacterially expressed His-tagged CDC-42 variants that mimic the GTP- and GDP-bound states (Q61L and T17N, respectively), we found that the SRGP-1 GAP domain also specifically interacts with the active form of CDC-42 (Figure 1b). This is consistent with previous findings, where CDC-42 (Cdc42 in mammals) activity is regulated by SRGP-1 (srGAP).24 Based on these findings, we concluded that SRGP-1, in addition to CED-10, might also regulate engulfment signaling through the Rho GTPase family member CDC-42 in C. elegans.
cdc-42 is required for embryonic cell corpse clearance
Multiple cdc-42 mutants are available for genetic analysis. A large deletion mutation cdc-42(gk388) completely eliminates the first coding exon and is likely a null allele (Supplementary Figure S2; also see Welchman et al.25). Although CDC-42 has many important functions in the early embryo, homozygous cdc-42(gk388) mutants (generated from heterozygous cdc-42/+ mothers, m+z−) develop up to the L3/L4 larval stages before arresting development because of significant maternal contribution of wild-type cdc-42 mRNA to the embryo. Thanks to this maternal rescue of early embryos, we could use this allele to score persistent cell corpse numbers in freshly hatched L1 larval heads. Homozygous m+z− cdc-42(gk388) animals only showed a mild persistent cell corpse phenotype in L1 larval and late embryos, possibly because of the maternally contributed cdc-42 mRNA (Table 1, Supplementary Figure S4A). Consistent with this hypothesis, progeny from cdc-42(RNAi)-treated mothers showed a much stronger engulfment defect (Supplementary Figure S4B).
cdc-42 acts in parallel to the ced-2, ced-5, ced-12 and mig-2 pathway
To better define the function of CDC-42 in engulfment signaling, we generated double mutants between cdc-42 and different canonical engulfment mutants. Consistent with our previous cdc-42(RNAi) results (Figure 1), cdc-42(gk388) significantly enhanced the persistent cell corpse phenotype of ced-2, ced-5, ced-12 and ced-10 mutants (Table 1). These results suggest that CDC-42 acts in parallel to the CED-2/5/12 module and its downstream CED-10 GTPase, or possibly upstream of CED-10, as the ced-10 mutants used here are not null.
We also analyzed the genetic interaction of cdc-42 with mig-2, the other small GTPase involved in cell corpse clearance. cdc-42(gk388) significantly enhanced the persistent cell corpse phenotype of mig-2(mu28) loss-of-function (lf) mutants in a sensitized ced-2(n1994) background. Conversely, the mig-2(gm103) gain-of-function (gf) mutation (which leads to activation of the CED-5–CED-12 GEF) decreased corpse numbers in ced-1; cdc-42 double mutants (Table 1). These results are consistent with a model in which cdc-42 and mig-2 act in parallel to each other.
cdc-42 likely acts downstream of the ced-1, ced-6 and ced-7 pathway
Next, we performed double mutant analyses with strong loss-of-function alleles of existing engulfment mutants of the ced-1/6/7 pathway. In contrast to the analysis described above, mutations in this pathway (ced-1, ced-6, ced-7 and ttr-52) were not enhanced by loss of cdc-42 function (Table 1). Importantly, loss of cdc-42 failed to enhance corpse persistence in ced-1; ced-5 double mutants. This indicates that an active CED-1/6/7 pathway is required for CDC-42 to promote engulfment in the absence of the CED-5–CED-12 complex. The most likely explanation for these combined observations is that CDC-42 functions in parallel to ced-5/12, possibly downstream of the ced-1/6/7 signaling cascade.
Overexpression of CDC-42 indicates that cdc-42 acts downstream of the ced-1/6/7 pathway
To confirm these results, we created three stable transgenic lines that drive the inducible (i.e., heat shock-triggered) expression of GFP-tagged, constitutively active (GTP-bound) CDC-42, CED-10 and RAC-2 GTPases (gfp::cdc-42(gf), gfp::ced-10(gf) and gfp::rac-2(gf)). We crossed these transgenic lines into the same engulfment-deficient mutants as in our epistatic analyses, and scored L1 head corpse numbers following heat shock treatment (Figure 2 and Supplementary Figure S3). Overexpression of cdc-42(gf) resulted in a suppression of ced-1, ced-6 and ced-7 mutants to almost wild-type levels. We observed a similar yet weaker effect when expressing gfp::ced-10(gf). In contrast, expression of gfp::rac-2(gf) failed to suppress such mutants at all. We therefore conclude that cdc-42, like ced-10, probably acts downstream of the ced-1/6/7 signaling cascade.
Overexpression of cdc-42 suppresses the engulfment defect of ced-1/6/7 mutants. (a) The engulfment defect of ced-1/6/7 but not ced-2/5/12 mutants is suppressed by cdc-42 overexpression. (b) cdc-42 overexpression requires a functional ced-2/5/12 pathway for ced-1/6/7 suppression. Worms were heat shocked for 90 min at 33°C, followed by an incubation at 20°C for 5 h to allow for expression of GFP::CDC-42 (gain of function). Persistent cell corpses were scored in the head of freshly hatched L1 larvae of the indicated genotypes. Alleles used: opIs280[Phsp-16.41::gfp::cdc-42(Q61L, gf); unc-119(+)], tiam-1(tm1556), ced-1(e1735), ced-6(n1813), ced-7(n1996), ced-2(n1994), ced-5(n1812), ced-12(k149), ced-10 (n3246) (strong loss of function) and ced-10(n1993) (weak loss of function). The opIs280 transgenic strains marked with an asterisk (*) also carry the unc-119(ed3) mutation that was used to follow (and is rescued by) the opIs280 transgene
In contrast, overexpression of cdc-42(gf) did not change persistent cell corpse numbers in mutants of the ced-5–ced-12 GEF complex, nor did cdc-42(gf) suppress the strong loss-of-function allele ced-10(n3246). Although the weak but viable allele ced-10(n1993) was partially suppressed by cdc-42(gf), this suppression was CED-5 dependent (Figure 2b). Moreover, we failed to observe any change in cell corpse numbers upon cdc-42(gf) overexpression in a ced-1; ced-5 or ced-6; ced-2 mutant background (Figure 2b). Taken together, these results indicate that overexpression of activated CDC-42 can compensate for a defect in the CED-1/6/7 pathway and CED-10, but only in the presence of an active CED-2/5/12 pathway.
CDC-42 is broadly expressed and accumulates around apoptotic cell corpses
Localization and function of CDC-42 have been mainly studied in one- and two-cell embryos, where CDC-42 participates in the regulation of cell polarity and spindle orientation.26, 27 In order to address the expression pattern of CDC-42 at later developmental stages, we created a transgene containing the genomic cdc-42 coding sequence under its endogenous 5′ and 3′ regulatory sequences fused N-terminally to GFP (opIs295[Pcdc-42::gfp::cdc-42::3′UTRcdc-42]; Figure 3a). The opIs295 transgene fully rescues the cdc-42(gk388) lethality and fertility defects. Furthermore, GFP::CDC-42 localization in the one-cell embryo resembles previously described expression patterns27, 28 (Figure 3b). We thus conclude that opIs295 is likely a functional reporter line.
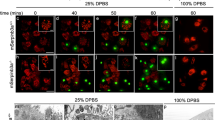
CDC-42 is membrane associated and accumulates around apoptotic cell corpses in embryos. (a) Schematic of the rescue construct opIs295. A 2.8-kb sequence upstream of the predicted start codon was used as a promoter to drive the expression of an N-terminal gfp::cdc-42(genomic) fusion product, followed by a 1-kb 3′ fragment that includes the cdc-42 3′ UTR. (b) Localization of CDC-42 to the anterior cortex in one-cell embryos. (c) In two-cell embryos, CDC-42 is enriched at the cortex of AB/P cell boundary. (d–f) CDC-42 expression pattern in early 24-cell (d, 80 min post fertilization (m.p.f.)), early twofold (e, 440 m.p.f.) and fourfold (f, 600 m.p.f.) embryos. CDC-42 is mainly localized to the cell cortexes and the cytosol. Representative apoptotic cell corpses are shown in magnified insets (e and f, arrowheads). Asterisk indicates nerve ring (e and f). Fluorescence channel (left), DIC (right). (g–i) CDC-42 accumulates at membranes around apoptotic cell corpses. (g) Confocal image of a 1.5-fold embryo with indicated area magnified to the right. (h) Plotted signal intensities along the line shown in (g), indicated by bn, mn and cn (background, membranes and membranes next to cell corpse, respectively). (i) Quantification of CDC-42 signal intensities. In different embryos, 12 different apoptotic cell corpses were analyzed. Data are shown as average±S.D. Genotype used: cdc-42(gk388) opIs295[Pcdc-42::gfp::cdc-42]. In all images, anterior is to the left, dorsal on top. Scale bar, 10 μm
We next used the opIs295 reporter to assess CDC-42 localization during embryonic development. In 2-cell to 32-cell embryos, we found CDC-42 mainly localized to cortexes at cell–cell contacts (Figures 3b and c). At late stages of embryonic development, we also observed a widespread CDC-42 expression in neuronal tissues such as the nerve ring, supporting the involvement of CDC-42 in neuronal processes24 (Figures 3d and e). During larval development and in adults, CDC-42 localized to cortexes in all cells, for example, in the vulva and in the tail (Supplementary Figures S5A–C). We therefore concluded that CDC-42 is very broadly expressed in C. elegans.
Previous work has shown that CED-1 and CED-6 in engulfing cells are selectively recruited to the plasma membrane surrounding apoptotic cells, generating a ‘halo’ pattern around the corpses.8, 10 We observed a similar accumulation of GFP::CDC-42 around apoptotic cell corpses during all developmental stages (Figures 3d–f, Supplementary Figures S5D and S6A). Importantly, CDC-42 was significantly enriched at membranes surrounding apoptotic cell corpses compared with cortical basal as well as background levels (Figures 3g and h). This was confirmed by GFP intensity quantification using confocal laser microscopy to measure background signal (bn), the signals at common membrane cortexes (mn) and at membranes surrounding cell corpses (cn), respectively (Figure 3i).
PAT-2/PAT-3 integrin, UNC-112 and the CDC-42 GEF UIG-1 drive CDC-42 recruitment to membranes surrounding cell corpses
We next wanted to know what signaling pathway directed CDC-42 membrane recruitment. Hsieh et al.23 recently described the CDC-42 GEF UIG-1 that promotes engulfment.23 UIG-1 is an UNC-112-interacting protein and colocalizes with UNC-112.29 Therefore, we asked whether uig-1 and unc-112 were involved in the regulation of engulfment. Loss of uig-1 function led at best to a mild increase, if any, of persistent cell corpses on its own (Figure 4 and Supplementary Table S1). As was the case for cdc-42, loss of uig-1 markedly enhanced the engulfment defect of ced-5, but not ced-1 mutants (Table 1). Because strong unc-112 mutants are very sick and develop extremely slowly, we performed our unc-112 epistatic studies in a dim-1 mutant background30 that suppresses the developmental delay of unc-112 mutants but has no visible effect by itself on engulfment (Table 1). Loss of unc-112 resulted in an engulfment defect in embryos. RNAi against cdc-42 failed to further enhance the engulfment defect of unc-112 mutant (Figure 4 and Supplementary Table S1). In contrast, persistent cell corpse numbers were significantly increased both in ced-1; unc-112 and ced-5; unc-112 double mutants (Table 1). These data are consistent with a model in which CDC-42 acts downstream of UNC-112 and UIG-1 in the clearance of apoptotic cell corpses.
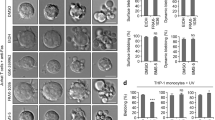
CDC-42 accumulation around apoptotic cell corpses depends on PAT-2 signaling. (a and c) Visualization and quantification of CDC-42 around somatic cell corpses in freshly hatched L1 larvae. (a) GFP::CDC-42 accumulates efficiently around cell corpses in L1 heads of ced-1 and ced-5 mutants. Representative apoptotic cell corpses are shown in magnified insets. (c) CDC-42 accumulation around apoptotic corpses does not depend on the CED-2/5/12 or CED-1/6/7 pathways. (b and d) Visualization and quantification of CDC-42 around somatic cell corpses in 1.5-fold stage embryos. (b) GFP::CDC-42 accumulates efficiently around cell corpses in 1.5-fold embryos in wild-type animals. (d) CDC-42 accumulation around apoptotic corpses depends on unc-112, uig-1, pat-2 and pat-3 signaling. Quantification of cell corpse numbers and GFP::CDC-42 coverage, shown in %, calculated as [GFP::CDC-42 halos]/[total corpse numbers] × 100. The absolute numbers of corpses are shown by light color on the left side, and the percentages are shown by dark color on the right side. Error bars on the right are only for the GFP::CDC-42 halos. Alleles used: ced-1(e1735), ced-2(n1994), ced-5(n1812), ced-6(n1813), ced-7(n1996), ced-10(n1993), ced-12(k149), dyn-1(n4039), unc-112(r367), dim-1(gk54), uig-1 (ok884), pat-2(ok2148), pat-3(st564), ina-1(gm39) and opIs295 [Pcdc-42::gfp::cdc-42(genomic)::3'UTRcdc-42; unc-119(+)]. Scale bar, 10 μm
In wild-type embryos, approximately one-quarter of cell corpses visible by DIC microscopy (Leica DM6000 B, Mannheim, Germany) are surrounded by a CDC-42::GFP halo (Figure 4). Mutations in the ced-1/6/7 and ced-2/5/12 pathways increased the number of cell corpses visible under DIC optics, but did not greatly affect the fraction of halo-positive corpses (25–35%; Figure 4). In contrast, loss of unc-112 and uig-1 function greatly reduced the number of halo-positive corpses (8%; Figure 4). Hsieh et al.23 recently reported that integrin-α PAT-2 can activate a CDC-42-dependent engulfment signaling pathway in embryonic muscle cells.23 Consistent with these observations, we found that CDC-42::GFP halos were also greatly reduced in pat-2 (α-integrin) and pat-3 (β-integrin) mutants (10% and 8%, respectively).
The presence of CDC-42 halos in internalization-defective mutants suggests that CDC-42 recruitment is an early process during corpse recognition. Consistent with this hypothesis, kinetic studies revealed that CDC-42::GFP accumulates significantly more frequently around early apoptotic corpses than around late corpses (Supplementary Figure S6B). We also noticed that a similar fraction of corpses in the L1 stage, at which point all corpses would be considered as ‘late’, as in embryos were labeled by CDC-42::GFP in ced-1 mutants (Figures 4c and d).
Taken together, our findings suggest that upon recognition of a neighboring dying cell, engulfing cells use a PAT-2/PAT-3-dependent signaling pathway, which includes UNC-112 and UIG-1, to recruit CDC-42 to the plasma membrane surrounding the corpse, possibly to help polarize the engulfing cell toward its prey.
The RacGEF TIAM-1 promotes cell corpse engulfment
How does CDC-42 influence cell corpse engulfment? Recently, Demarco et al.31 reported that the Rac GEF protein TIAM-1 acts downstream of CDC-42 and upstream of CED-10/Rac1 in neuronal protrusion and axon guidance, offering a concrete way for CDC-42 to influence cytoskeletal rearrangement. Several lines of observations suggest that TIAM-1 also plays a role in cell corpse engulfment. First, whereas tiam-1 single mutants did not show any persistent cell corpses (Supplementary Table S1), loss of tiam-1 function increased the engulfment defect of weak ced-10 mutants (Table 1). Second, we found that in the absence of TIAM-1 activity, overexpression of CDC-42 failed to suppress the engulfment defect in ced-10 mutants (Figure 2). Finally, we observed that whereas loss of tiam-1 did not increase the engulfment defect of cdc-42(lf) or cdc-42(RNAi) animals, tiam-1; cdc-42; ced-10 triple mutant animals contained more corpses than both tiam-1; ced-10 and cdc-42; ced-10 double mutants (Table 1 and Supplementary Table S1). These results are consistent with a role of TIAM-1 either in parallel to or downstream of CDC-42 in cell corpse clearance.
cdc-42 promotes cell killing
We and others have previously shown that engulfment can also kill cells on the verge of death.3, 22, 32, 33 To test whether CDC-42 can also promote the removal of living cells, we measured P1, P2 and P9–12 Pn.aap cell survival in the ventral nerve cord of L3 larvae. In wild-type animals, these cells, which can be visualized with the Plin-11:gfp reporter nIs96, undergo programmed cell death during early larval development. In engulfment-deficient mutants, a significant fraction of those cells survive and remain alive during adulthood (Supplementary Figure S7).32, 34 As is the case for other engulfment mutants such as ced-1, ced-5 and ced-10, the cdc-42(gk388) animals showed extra Pn.aap surviving cells compared with wild-type controls (Supplementary Figure S7). This effect was particularly pronounced in a sensitized ced-3(n2438) reduction-of-function background. These observations support an involvement of cdc-42 in the recognition and elimination of subviable cells in C. elegans.
Discussion
Rho GTPase family members are known to drive cytoskeletal rearrangements in several processes, such as phagocytosis, cell migration and invasion, integrin-mediated cell adhesion and spreading.35, 36, 37 In C. elegans, two Rho GTPase family members, CED-10/Rac1 and MIG-2/RhoG, have previously been shown to regulate apoptotic corpse clearance. Here, we show that a third small GTPase, CDC-42, also plays an important role in this process. Our genetic analysis suggests that cdc-42 acts in parallel to or downstream of the ced-1/6/7 module and in parallel to the ced-2/5/12 module, possibly upstream or in parallel to ced-10 (Figure 2, Table 1 and Supplementary Table S1). Recruitment of CDC-42 to the plasma membrane facing apoptotic cells depends on PAT-2/PAT-3 integrin, UNC-112 and the CDC-42 GEF UIG-1, but not the canonical ced-1/6/7 or ced-2/5/12 modules (Figure 4). Our observations confirm and greatly expand on a recent publication by Hsieh et al.23 that showed that integrin-α PAT-2 might recognize exposed PS on apoptotic cells and regulate corpse engulfment through UIG-1 and CDC-42 in C. elegans muscle cells.
What is the function of CDC-42 in apoptotic cell removal? Corpse clearance in C. elegans is commonly divided into three main steps: recognition of the neighboring apoptotic cell, corpse internalization/engulfment and phagosome maturation.9 Rac GTPase CED-10 promotes the rearrangement of the cytoskeleton that is required to drive formation of the phagocytic cup and corpse internalization. Previous studies in C. elegans showed that CDC-42 plays an important role in the establishment and maintenance of cell polarity in early embryos.27, 38, 39, 40 In mammals, Cdc42 participates in the regulation of various processes including cell polarity, cell migration, phagocytosis and endomembrane trafficking.41, 42, 43, 44 Cdc42 has also been implicated in the integrin and Fc receptor-mediated internalization of apoptotic corpses by macrophages.45, 46
Our genetic analysis and the role of CDC-42 in cell polarity suggest a model in which corpse internalization includes two distinct engulfment activities: in this model an early polarization toward the apoptotic corpse is later followed by cytoskeletal remodeling activities that drive phagocytic cup formation. Our data suggest that apoptotic cells are recognized via at least two receptor pathways (Figure 5): PAT-2/3 integrins, which recruit CDC-42 to the plasma membrane via UNC-112 and UIG-1, and CED-1, which is also enriched around apoptotic corpses independently of CDC-42 (data not shown). Based on the known role of CDC-42 in the control of cell polarity in other processes, we postulate that active CDC-42, in response to CED-1/6/7 pathway activation, drives the subsequent establishment of a dynamic asymmetry in the distribution or activation of the cytoskeletal rearrangement machinery, including the CED-5–CED-12 GEF and its target CED-10/Rac1, ultimately leading to phagocytic cup formation around the recognized corpse (Figure 5). Malfunctioning polarization (i.e., in ced-1/6/7 mutants) still allows cytoskeletal remodeling to take place, but likely in a less efficient/directional way. An impaired cytoskeletal remodeling machinery (i.e., in ced-2/5/12 mutants) leads to an engulfing cell that still polarizes toward the corpse but engulfs it only poorly (probably through a less-efficient back-up signaling cascade acting in parallel). Finally, interfering with both polarity and cytoskeletal remodeling results in an additive phenotype.
cdc-42 regulates engulfment in response to PAT-2/CED-1–UIG-1 signaling. Genetic model of cell corpse clearance in C. elegans. cdc-42 acts downstream of pat-2/3, downstream of or in parallel to the ced-1/6/7 pathway. See text for details. Components involved in phagosome maturation are not shown. GTPases, GEFs and GAP are shown in gray, orange and blue boxes, respectively. Dashed arrows indicate potential regulation
Such a model can explain many of our observations. First, overexpression of CDC-42 might make its activation independent of the ced-1/6/7 pathway, explaining why such an overexpression rescues ced-1/6/7 pathway mutants. In contrast, overexpression of CDC-42 cannot efficiently drive phagocytic cup formation, and hence cannot suppress ced-2/5/12 mutants. Second, cdc-42(lf) would not enhance ced-1/6/7 pathway mutants (as CDC-42 activation and cell polarization is already disrupted in these mutants) but would enhance mutants involved in phagocytic cup formation (ced-2/5/12). Finally, hyperactivation of the pathway leading to phagocytic cup formation could be expected to compensate for defects in cell polarization, whereas the opposite would not be true – which is indeed what is experimentally observed. For example, we found that mig-2(gf) mutants fail to suppress mutants of the ced-5–ced-12 GEF complex, but can rescue ced-1 or ced-1;cdc-42 double mutants. Finally, overexpression of ced-10 has been shown to rescue engulfment mutants in both ced-1/6/7 and ced-2/5/12 pathways.
An alternative function for CDC-42 in phagocytosis has recently been suggested by Mohammadi and Isberg,47 who showed that in mammalian cells, Cdc42 is required to drive the exocytosis of recycling vesicles, likely to generate the additional plasma membrane required for phagocytic cup formation. Overexpression of the small GTPase Rab11 could rescue the large particle uptake defect in Cdc42-depleted cells, identifying RAB-11 as a potential downstream regulator of CDC-42.47
Our model however also leaves several questions unanswered. For example, how CED-1/6/7 might mediate UIG-1-dependent membrane recruitment and activation of CDC-42 remains to be determined. Downstream targets of CDC-42 beyond TIAM-1 also remain to be identified. Finally, it remains unclear why loss of CDC-42 function on its own only leads to a mild engulfment defect. One possibility is that CDC-42 function is required only in a subset of engulfing cells. Consistent with this hypothesis, Hsu and colleagues23, 48 reported the existence of a second integral signaling pathway, activated by INA-1, that acts in parallel to PAT-2/3 and activates the ced-2/-5/-12/-10 signaling module via the tyrosine kinase SRC-1. Different cell types might thus use different integrins, leading to the activation of distinct signaling pathways that trigger similar cellular processes.
The involvement of cdc-42 in apoptotic cell clearance by the phagocytic cell expands the already broad variety of conserved developmental processes such as asymmetric cell division, cell migration, epithelial remodeling and nervous system development in which CDC-42 plays a key role.39 Given the evolutionarily conserved function of the engulfment machinery, it is likely that Cdc42 promotes cell corpse clearance in similar ways in mammals and C. elegans. Using C. elegans to further dissect cdc-42 signaling mechanisms will provide new insights into the mechanism of apoptotic cell clearance in humans, a process that has been associated with a variety of human diseases.1
Materials and Methods
Mutations/strains used
C. elegans strains were grown at 20°C as previously described. Wild type: used was Bristol N2. The alleles used were as follows: LGI: tiam-1(tm1556), ced-12(k149) and ced-1(e1735); LGII: cdc-42(gk388); LGIII: ced-6(n1813), ced-6(tm1826), ced-7(n1996), pat-2(ok2148), pat-3(st564), ina-1(gm39), ttr-52(sm211) and unc-119(ed3); LGIV: ced-2(e1752), ced-2(n1994), ced-2(op327), ced-10(n1993), ced-10(t1875), ced-5(n1812), ced-5(tm1949) and srgp-1(ok300); LGV: uig-1(ok884) and unc-112(r367); LGX: mig-2(mu28), mig-2(gm103gf) and dim-1(gk54). Unless otherwise noted, all alleles are described (http://www.wormbase.org/). Integrated arrays were: opIs280[Phsp-16.41::gfp::cdc-42(gf)::3′UTRlet-858; unc-119(+)], opIs295[Pcdc-42::gfp::cdc-42(genomic)::3′UTRcdc-42; unc-119(+)] II, opIs296[Pcdc-42::cfp::cdc-42(genomic)::3′UTRcdc-42; unc-119(+)],opIs135[Pced-1::ced-1(genomic)::yfp::3′UTRced-1; unc-69(+)] and nIs96[Plin-11::gfp] V.Balancers were as follows: mIn1(dpy-10(e128); mIs14[myo-2::gfp; pes-10::gfp]) (II), hT2[bli-4(e937) let-???(q782) qIs48](I;III) and nT1(qIs51[myo-2::gfp; pes-10::gfp]) (IV;V).
Phenotypic analysis
Larval L1 head apoptotic cell corpses: Mixed culture plates were washed off with M9 several times to remove everything except eggs. After 45 min, freshly hatched L1 were mounted on a 3% agar pad, anesthetized (2.5 mM levamisole in M9) and immediately scored for persistent cell corpses in the head region (i.e., anterior from the posterior bulb) using a DIC microscope. Germ cell corpses: More than 20 either 12 or 24 h post L4/adult molts were mounted on a 3% agar slide and anesthetized in a droplet of 5 mM levamisol in M9. Refractive apoptotic germ cell corpses were scored using the same microscope, followed if applicable by fluorescent halo scoring (of strong fluorescent reporters), under the corresponding epifluorescence channel of the microscope described above. Gonads of weak fluorescent germline reporter strains were dissected in PBS and mounted on 3% agar slides. Pn.aap cell survival: The number of surviving Pn.aap cells (P1, P2, P9–P12.aap) were scored in nIs96[Plin-11::gfp] L3 or early L4 larvae under an M2Bio epifluorescence dissecting microscope (Zeiss, Hamburg, Germany).32 Heat shock treatment: Mixed culture plates were heat shocked for 90 min at 33°C. After 5 h, worms were washed off the plates, and 50 min later freshly hatched L1 larvae were scored for persistent cell corpses in the head region.
RNAi by feeding
RNAi was performed as described previously49 with the following modifications: NGM-agarose plates containing 2 mM IPTG were seeded with 250 μl of appropriate bacterial clones 12 h before the addition of worms. Approximately 30 staged L1 larvae of the corresponding genotype were seeded in triplicates on plates and grown at 20°C. For rac-2, ced-10, crp-1 and chw-1 RNAi, staged animals (P0) were grown on RNAi plates and freshly hatched L1 progeny larvae (F1) were scored for corpse numbers in the head region. For cdc-42(RNAi), staged P0 L3 larvae were used instead of L1 larvae. No condition applied for rho-1(RNAi) led to viable F1 larvae.
Total RNA isolation and cDNA synthesis
Mixed worm cultures from two 9 cm plates were washed off with M9, rinsed twice with M9 and total RNA was extracted as described previously.50 The dry total RNA was resuspended in 50 μl ddH2O and similar amounts used for cDNA synthesis according to the manufacturer’s instructions (Super Script III, Invitrogen, Carlsbad, CA, USA).
Generation of transgenic strains
Transgenic worms (opEx and opIs alleles) were generated by microparticle bombardment in a Biolistic Particle Delivery System (PDS-1000, Bio-Rad, Hercules, CA, USA) as described previously.22 As a transformation marker, unc-119(ed3) was used.
In vitro GTPase pulldowns
In vitro pulldowns were performed as described previously.22 Briefly, C. elegans GTP- and GDP-binding GTPase isoform (QXXL and TXXN) expressing plasmids were transformed into BL21(DE3)pLysS (Fisher Scientific, Hampton, NH, USA) and protein expression was induced by 1 mM IPTG at 37°C. All His-tagged GTPase proteins were purified using His-Bind resin (Novagen, Darmstadt, Germany) according to the manufacturer’s instructions. All of the buffers contained 2 mM MgCl2 and 1 mM Tris(Hydroxypropyl)Phosphine (THP). The proteins were dialyzed against 10 mM Tris-HCl (pH 7.5), 10 mM NaCl, 2 mM MgCl2 and 0.1 mM THP, and snap frozen at −80°C. GST and GST::GAP domain isoform containing plasmids were transformed into BL21(Gold) (Fisher Scientific) and their expression products purified and immobilized onto Glutathione Sepharose beads by standard methods. Immobilized GST fusion proteins (30 μg) were incubated with 10 μg of His-tagged GTPase proteins in a buffer containing 10 mM Tris-HCl (pH 7.9), 100 mM NaCl, 2 mM MgCl2 and 1 mM THP, 0.5% NP-40 and 10% glycerol. The incubation was carried out for 2 h at 4°C with agitation. The beads were washed in the same buffer three times and the proteins were separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). His-tagged proteins were immunoblotted with rabbit anti-His antibodies (Santa Cruz, Santa Cruz, CA, USA; 1 : 1000).
4D microscopy
The 4D microscopy was preformed as previously described.34, 51 Briefly, young embryos were isolated and mounted on agarose slides in M9 and sealed by vaseline. The Z-stacks of embryos were taken every minute using DIC microscope as described before during early embryonic development (∼4 h). Persistent cell corpses were scored at indicated stages of embryonic development.
Primers and plasmids
Primers and plasmids are listed in Supplementary Table S2. Plasmids were fully sequenced before microparticle bombardment or expression in bacteria. If not mentioned otherwise, the following ‘LazyBoy’ starting plasmids were used: pLN022[SbfI promoter AscI gene FseI reporter PacI let-8583′UTR ApaI, unc-119(+)] or pLN019[SbfI promoter AscI reporter AscI gene FseI let-8583′UTR ApaI, unc-119(+)].
Abbreviations
- cdc :
-
cell division control
- ced :
-
cell death abnormal
- pat :
-
paralyzed arrest at twofold
- GAP:
-
GTPase-activating protein
- GEF:
-
guanosine exchange factor
- gf :
-
gain of function
- lf :
-
loss of function
- mig :
-
migration defective
- RNAi:
-
RNA interference
- Srgp-1 :
-
Slit/Robo GTPase activating protein 1
- UTR:
-
untranslated region
References
Nagata S, Hanayama R, Kawane K . Autoimmunity and the clearance of dead cells. Cell 2010; 140: 619–630.
Fullard JF, Kale A, Baker NE . Clearance of apoptotic corpses. Apoptosis 2009; 14: 1029–1037.
Hoeppner DJ, Hengartner MO, Schnabel R . Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 2001; 412: 202–206.
Schwartz HT . A protocol describing pharynx counts and a review of other assays of apoptotic cell death in the nematode worm Caenorhabditis elegans. Nat Protoc 2007; 2: 705–714.
Ellis RE, Horvitz HR . Two C. elegans genes control the programmed deaths of specific cells in the pharynx. Development 1991; 112: 591–603.
Zhou Z, Caron E, Hartwieg E, Hall A, Horvitz HR . The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev Cell 2001; 1: 477–489.
Gumienny TL, Hengartner MO . How the worm removes corpses: the nematode C. elegans as a model system to study engulfment. Cell Death Differ 2001; 8: 564–568.
Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H et al. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 2005; 434: 93–99.
Pinto SM, Hengartner MO . Cleaning up the mess: cell corpse clearance in Caenorhabditis elegans. Curr Opin Cell Biol 2012; 24: 881–888.
Zhou Z, Hartwieg E, Horvitz HR . CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 2001; 104: 43–56.
Wang X, Li W, Zhao D, Liu B, Shi Y, Chen B et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol 2010; 12: 655–664.
Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol 2007; 9: 541–549.
Wu YC, Horvitz HR . The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 1998; 93: 951–960.
Liu QA, Hengartner MO . Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell 1998; 93: 961–972.
Yu X, Odera S, Chuang C-H, Lu N, Zhou Z . C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev Cell 2006; 10: 743–757.
Yu X, Lu N, Zhou Z . Phagocytic receptor CED-1 initiates a signaling pathway for degrading engulfed apoptotic cells. PLoS Biol 2008; 6: e61.
Reddien PW, Horvitz HR . CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol 2000; 2: 131–136.
Wu YC, Horvitz HR . C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 1998; 392: 501–504.
Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY . C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 2001; 1: 491–502.
Akakura S, Kar B, Singh S, Cho L, Tibrewal N, Sanokawa-Akakura R et al. C-terminal SH3 domain of CrkII regulates the assembly and function of the DOCK180/ELMO Rac-GEF. J Cell Physiol 2005; 204: 344–351.
Hurwitz ME, Vanderzalm PJ, Bloom L, Goldman J, Garriga G, Horvitz HR . Abl kinase inhibits the engulfment of apoptotic [corrected] cells in Caenorhabditis elegans. PLoS Biol 2009; 7: e99.
Neukomm LJ, Frei AP, Cabello J, Kinchen JM, Zaidel-Bar R, Ma Z et al. Loss of the RhoGAP SRGP-1 promotes the clearance of dead and injured cells in Caenorhabditis elegans. Nat Cell Biol 2011; 13: 79–86.
Hsieh H-H, Hsu T-Y, Jiang H-S, Wu Y-C . Integrin α PAT-2/CDC-42 signaling is required for muscle-mediated clearance of apoptotic cells in Caenorhabditis elegans. PLoS Genet 2012; 8: e1002663.
Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 2001; 107: 209–221.
Welchman DP, Mathies LD, Ahringer J . Similar requirements for CDC-42 and the PAR-3/PAR-6/PKC-3 complex in diverse cell types. Dev Biol 2007; 305: 347–357.
Schonegg S, Hyman AA . CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development 2006; 133: 3507–3516.
Kumfer KT, Cook SJ, Squirrell JM, Eliceiri KW, Peel N, O'Connell KF et al. CGEF-1 and CHIN-1 regulate CDC-42 activity during asymmetric division in the Caenorhabditis elegans embryo. Mol Biol Cell 2010; 21: 266–277.
Motegi F, Sugimoto A . Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol 2006; 8: 978–985.
Hikita T, Qadota H, Tsuboi D, Taya S, Moerman DG, Kaibuchi K et al. Identification of a novel Cdc42 GEF that is localized to the PAT-3-mediated adhesive structure. Biochem Biophys Res Commun 2005; 335: 139–145.
Rogalski TM, Gilbert MM, Devenport D, Norman KR, Moerman DG . DIM-1, a novel immunoglobulin superfamily protein in Caenorhabditis elegans, is necessary for maintaining bodywall muscle integrity. Genetics 2003; 163: 905–915.
Demarco RS, Struckhoff EC, Lundquist EA . The Rac GTP exchange factor TIAM-1 acts with CDC-42 and the guidance receptor UNC-40/DCC in neuronal protrusion and axon guidance. PLoS Genet 2012; 8: e1002665.
Reddien PW, Cameron S, Horvitz HR . Phagocytosis promotes programmed cell death in C. elegans. Nature 2001; 412: 198–202.
Galvin BD, Kim S, Horvitz HR . Caenorhabditis elegans genes required for the engulfment of apoptotic corpses function in the cytotoxic cell deaths induced by mutations in lin-24 and lin-33. Genetics 2008; 179: 403–417.
Sulston JE, Horvitz HR . Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 1977; 56: 110–156.
Price LS, Leng J, Schwartz MA, Bokoch GM . Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 1998; 9: 1863–1871.
Niedergang F, Chavrier P Regulation of phagocytosis by RhoGTPases. In: Boquet P, Lemichez E (eds). Bacterial Virulence Factors and Rho GTPases. Springer: Berlin, Heidelberg, 2005 pp 43–60 available at http://link.springer.com/chapter/10.1007/3-540-27511-8_4.
Partridge MA, Marcantonio EE . Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell 2006; 17: 4237–4248.
Kay AJ, Hunter CP . CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Curr Biol 2001; 11: 474–481.
Anderson DC, Gill JS, Cinalli RM, Nance J . Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science 2008; 320: 1771–1774.
Beatty A, Morton DG, Kemphues K . PAR-2, LGL-1 and the CDC-42 GAP CHIN-1 act in distinct pathways to maintain polarity in the C. elegans embryo. Development 2013; 140: 2005–2014.
Caron E, Hall A . Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 1998; 282: 1717–1721.
Caron E . Phagocytosis: Rac and roll over the corpses. Curr Biol 2000; 10: R489–R491.
Hoppe AD, Swanson JA . Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 2004; 15: 3509–3519.
Kroschewski R, Hall A, Mellman I . Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1999; 1: 8–13.
Leverrier Y, Ridley AJ . Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol 2001; 11: 195–199.
Savill J, Dransfield I, Gregory C, Haslett C . A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965–975.
Mohammadi S, Isberg RR . Cdc42 interacts with the exocyst complex to promote phagocytosis. J Cell Biol 2013; 200: 81–93.
Hsu T-Y, Wu Y-C . Engulfment of apoptotic cells in C. elegans is mediated by integrin α/SRC signaling. Curr Biol 2010; 20: 477–486.
Kamath RS, Ahringer J . Genome-wide RNAi screening in Caenorhabditis elegans. Methods 2003; 30: 313–321.
Chomczynski P, Sacchi N . Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 156–159.
Neukomm LJ, Nicot AS, Kinchen JM, Almendinger J, Pinto SM, Zeng S et al. The phosphoinositide phosphatase MTM-1 regulates apoptotic cell corpse clearance through CED-5-CED-12 in C. elegans. Development 2011; 138: 2003–2014.
Acknowledgements
We thank the members of the Hengartner and Hajnal lab for discussions on this manuscript. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), the C. elegans Gene Knockout Consortium (Oklahoma, USA) and the National Bioresource Project (Japan). This work was funded by the Swiss National Science Foundation and by the canton of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by S Nagata
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Neukomm, L., Zeng, S., Frei, A. et al. Small GTPase CDC-42 promotes apoptotic cell corpse clearance in response to PAT-2 and CED-1 in C. elegans. Cell Death Differ 21, 845–853 (2014). https://doi.org/10.1038/cdd.2014.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.23
This article is cited by
-
Network analysis in aged C. elegans reveals candidate regulatory genes of ageing
Biogerontology (2021)
-
Pathogenetic basis of Takenouchi-Kosaki syndrome: Electron microscopy study using platelets in patients and functional studies in a Caenorhabditis elegans model
Scientific Reports (2019)
-
Programmed cell death and clearance of cell corpses in Caenorhabditis elegans
Cellular and Molecular Life Sciences (2016)