Abstract
WW domain-containing oxidoreductase (WWOX), a putative tumour suppressor, is suggested to be involved in the hyperphosphorylation of Alzheimer's Tau. Tau is a microtubule-associated protein that has an important role in microtubule assembly and stability. Glycogen synthase kinase 3β (GSK3β) has a vital role in Tau hyperphosphorylation at its microtubule-binding domains. Hyperphosphorylated Tau has a low affinity for microtubules, thus disrupting microtubule stability. Bioinformatics analysis indicated that WWOX contains two potential GSK3β-binding FXXXLI/VXRLE motifs. Immunofluorescence, immunoprecipitation and molecular modelling showed that WWOX interacts physically with GSK3β. We demonstrated biochemically that WWOX can bind directly to GSK3β through its short-chain alcohol dehydrogenase/reductase domain. Moreover, the overexpression of WWOX inhibited GSK3β-stimulated S396 and S404 phosphorylation within the microtubule domains of Tau, indicating that WWOX is involved in regulating GSK3β activity in cells. WWOX repressed GSK3β activity, restored the microtubule assembly activity of Tau and promoted neurite outgrowth in SH-SY5Y cells. Conversely, RNAi-mediated knockdown of WWOX in retinoic acid (RA)-differentiated SH-SY5Y cells inhibited neurite outgrowth. These results suggest that WWOX is likely to be involved in regulating GSK3β activity, reducing the level of phosphorylated Tau, and subsequently promoting neurite outgrowth during neuron differentiation. In summary, our data reveal a novel mechanism by which WWOX promotes neuronal differentiation in response to RA.
Similar content being viewed by others
Main
WW domain-containing oxidoreductase (WWOX/WOX1/FOR)1 is a 46 kDa protein that has been implicated in human tumourigenesis, nervous system development and Alzheimer's disease (AD).2, 3, 4 WWOX contains two N-terminal WW domains (containing conserved tryptophan residues) and one COOH-terminal short-chain alcohol dehydrogenase/reductase (ADH/SDR) domain. Both the WW and ADH domains mediate protein–protein interactions. The WWOX has been reported to bind to many proteins, including p53, p73, erythroblastic leukaemia viral oncogene homolog 4, ezrin, activator protein-2 γ, small integral membrane protein of the lysosome/late endosome, c-Jun N-terminal kinase 1 and mouse double mutant 2.5, 6 Although some studies have suggested that WWOX might have a critical role in regulating Tau hyperphosphorylation,2 the details of this regulation remain unclear.
Glycogen synthase kinase 3β (GSK3β), a serine/threonine kinase that was first identified based on its ability to phosphorylate glycogen synthase and regulate glycogen metabolism, is now known to be a key kinase in several important signalling pathways. The dysregulation of GSK3β is involved in several major human diseases, including diabetes, cancer, AD and bipolar disorder.7 Researchers have reported a number of proteins that associate with GSK3β and regulate its activity. The strongest examples is Axin, which are involved in the Wnt signalling pathway.2, 8 Chou et al.9 used the yeast two-hybrid method to identify a novel GSK3β interacting protein, GSKIP, which can bind to GSK3β and inhibit its kinase activity.
The role of GSK3β in regulating neural cell differentiation is controversial. GSK3β has been shown to facilitate neurite outgrowth by preventing E2F1 from inhibiting the transcription of the cell cycle inhibitors p21 and p15.10 However, other evidence has indicated that the inactivation of GSK3β results in collapsin response mediator protein 2 (CRMP-2) dephosphorylation, which leads to enhanced microtubule polymerisation and axon growth.11 Therefore, the function of GSK3β in the regulation of neural differentiation remains unclear.
Chen et al.12 reported that WWOX likely has a role in the development of the nervous system. They found that WWOX was differentially expressed during various stages of brain development in mice, indicating a potential role for WWOX in promoting neuronal differentiation and maturation.12 However, the molecular mechanism by which WWOX facilitates neural differentiation has yet to be revealed. In this study, we demonstrated that WWOX enhances retinoic acid (RA)-mediated neurite outgrowth and identified a novel mechanism through which WWOX promotes neurite outgrowth by interacting with and suppressing GSK3β kinase activity in the presence of RA. Our findings suggest that the regulation of GSK3β activity by WWOX has a vital role in RA-induced neural-cell differentiation.
Results
WWOX is required for neuronal cell differentiation
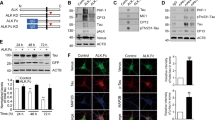
RA can induce neuroblastomal differentiation in SH-SY5Y cells.13, 14, 15 From 1 to 4 days after RA induction, SH-SY5Y cells progressively underwent phenotypic changes that were compatible with neuron-like morphology, characterised by neurite outgrowth (Figure 1a). The expression of the neuronal marker βIII-tubulin progressively increased over 4 days of RA treatment (Figure 1b). The expression of cyclin D1, a cell-cycle progression marker, gradually decreased during the RA treatment. Increased expression of WWOX was observed in the RA-treated SH-SY5Y cells, accompanying the decreased phosphorylation of Tau at both S396 and S404; phosphorylation at S422 was unchanged (Figures 1b and c). Since studies have shown that S404 and S396 may be phosphorylated by GSK3β in vivo,16 we also examined whether the phosphorylation level of GSK3β and its downstream target, β-catenin, are affected by RA treatment. We found that the phosphorylation levels of phospho-GSK3β S9 and phospho-β-catenin remained normal. We therefore suspected that the reduced GSK3β-mediated Tau phosphorylation and increased WWOX expression are indispensable for RA-induced SH-SY5Y cell differentiation. Furthermore, SH-SY5Y cells in which WWOX expression was reduced by RNAi showed increased pTau S396 levels and notably decreased neurite outgrowth (Figures 1d and e). Collectively, these results indicate that WWOX may have a vital role in SH-SY5Y cell differentiation and in Tau phosphorylation.
RA-induced SH-SY5Y differentiation is reduced by WWOX knockdown. (a) Morphological changes in SH-SY5Y neuroblastoma cells treated with or without RA (upper panel). The percentage of differentiated cells was determined for cells treated with 10 μM RA, and the photos were taken on days 0, 1, 2, 3 and 4 (lower panel). (b) Cells were treated with RA for the indicated times and collected for protein extraction. Western blots were conducted to measure the levels of pTau S404, pTau S422, pTauS396, Tau, WWOX, cyclin D1, phospho-GSK3βS9, GSK3β, β-catenin, phospho-β-catenin and actin. (c) Western blot results of Figure 2b were quantified by scanning the autoradiogram using a densitometer. (d) SH-SY5Y cells were transfected with two pre-designed siRNA targeting WWOX for 24 h, and the resulting cell lysates were subjected to western blot analysis. (e) SH-SY5Y cells were transfected with either control siRNA or WWOX siRNA as indicated. After 24 h of transfection, cells were washed, and the medium was replaced with 1% serum including 10 μM RA. Cells with neurites longer than twice the length of the cell body were counted. Data are presented as the mean±S.E. from three independent experiments. *P<0.05, **P<0.01 when compared with the mock control
WWOX interacts and colocalises with GSK3β in vivo
Hernández et al.16 demonstrated that S396 and S404 on Tau are GSK3β-dependent phosphorylation sites. Thus, we presumed that WWOX-dependent dephosphorylation of Tau occurred through an inhibition of GSK3β activity. We performed a bioinformatic analysis to align WWOX with well-known GSK3β inhibitors (Figure 2a) and found that WWOX296−320 and WWOX388−412 contain FXXXLI/VXRLE, a highly conserved GSK3β-binding motif within GSKIP115−139, Axin381−405, and FRAT205−229. Previous reports have shown that GID381−405, a 25-amino acid region of Axin, is critical for its association with GSK3β.8 Interestingly, WWOX296−320 and WWOX388−412 are similar to GID381−405 in Axin (Figure 2a). WWOX296−320 is 40% similar to Axin1381−405, 32% similar to Axin2363−387, 36% similar to FRAT1205−229 and 34% similar to GSKIP115−139. WWOX388−412 is 26% similar to Axin1381−405, 34% similar to Axin2363−387, 36% similar to FRAT1205−229 and 32% similar to GSKIP115−139 (Figure 2a). These results suggested that WWOX may interact with GSK3β. Hence, we performed immunofluorescence and immunoprecipitation experiments to determine whether WWOX is associated with GSK3β. First, SH-SY5Y cells were co-transfected with plasmids expressing GSK3β fused to green fluorescent protein (GFP) (GFP–GSK3β) and WWOX–HA to examine the in vivo association of WWOX and GSK3β. Confocal fluorescent microscopy demonstrated that WWOX and GSK3β colocalised in the cytoplasm of SH-SY5Y cells (Figure 2b). We then performed co-immunoprecipitation experiments to confirm the in vivo interaction between WWOX and GSK3β in SH-SY5Y cells. Cell extracts were prepared from SH-SY5Y cells that had been transiently transfected with the WWOX–HA construct. Figure 2c (left panel) shows that the anti-HA antibodies precipitated HA-tagged WWOX, and that endogenous GSK3β co-precipitated with the WWOX protein complex. Similarly, ectopic HA-tagged WWOX was co-immunoprecipitated by anti-GSK3β antibodies (Figure 2c, right panel).
WWOX physically interacts and colocalises with GSK3β in vitro and in vivo. (a) Upper panel: the amino acid sequence of WWOX 296−320, which is similar to highly conserved regions of Axin1381−405, Axin2363−387, FRAT1205−229 and GSKIP115−139, is highlighted in grey. Lower panel: the amino acid sequence of WWOX 388−412, which is similar to highly conserved regions of Axin1381−405, Axin2363−387, FRAT1205−229 and GSKIP115−139, is highlighted in grey. Identical amino acids are highlighted in black. (b) SH-SY5Y cells were co-transfected with pEGFP–GSK3β and pcDNA3.1–HA–WWOX constructs for 24 h. Cells were fixed with 3.7% formaldehyde, permeabilised, and incubated with mouse anti-HA primary antibodies and rhodamine-conjugated secondary antibodies. The cells were then observed using a Zeiss LSM510 confocal microscope. (c) Cells were harvested for immunoprecipitation using anti-WWOX (left) or anti-GSK3β (right) antibodies. WWOX-associated GSK3β was recognised by anti-GSK3β. Anti-HA was used to detect overexpressed HA-tagged WWOX. (d) SH-SY5Y cells were transiently transfected with the WWOX–HA construct and treated with RA for 24 h. The cells were harvested for immunoprecipitation with anti-GSK3β. Immunoprecipitated GSK3β was recognised by antibodies against GSK3β. Anti-HA was used to detect HA-tagged WWOX. (e) Mouse brain extract was subjected to immunoprecipitation using anti-WWOX or anti-GSK3β as indicated. The immunoprecipitated complex was separated by molecular weight using SDS-PAGE. Anti-WWOX or anti-GSK3β antibodies were used to detect WWOX or GSK3β on the PVDF membrane
We considered the possibility that WWOX could regulate Tau phosphorylation through direct interaction. In Supplementary Figure A, however, we show that although WWOX–HA can be immunoprecipitated with anti-HA antibodies, Tau was not co-immunoprecipitated in the complex (Supplementary Figure A), indicating that WWOX does not stably interact with Tau in SH-SY5Y cells. To clarify whether the interaction between WWOX and GSK3β is affected by RA treatment, we performed co-immunoprecipitation experiments in cells that had been stimulated with RA. SH-SY5Y cells were transiently transfected with the WWOX construct to mimic the upregulation of WWOX by RA treatment. Figure 2d shows that the amount of GSK3β co-immunoprecipitated by WWOX from RA-treated SH-SY5Y cells was similar to the amount precipitated in untreated controls. Subsequently, immunoprecipitation was performed in mouse brain extracts to verify the physiological interaction between WWOX and GSK3β. Figure 2e shows that both GSK3β and WWOX were precipitated by anti-GSK3β or anti-WWOX antibodies. These results indicate that WWOX physiologically associates and colocalises with GSK3β in vivo.
The WWOX–GSK3B interaction is mediated by a conserved motif in WWOX
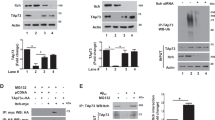
To narrow down the binding site on WWOX that is responsible for its binding to GSK3β, various WWOX functional domains, ww1 (1–60 a.a.), ww2 (40–110 a.a.), ww (1–110 a.a.) and ADH (110–414 a.a.), were expressed as glutathione S-transferase (GST) fusions in E. coli and affinity-purified on glutathione beads (Figure 3a). As shown in Figure 3b, the result of GST pull-down assay reveals an intense anti-GSK3β band was bound by the full-length WWOX and ADH-domain affinity matrix (Figure 3b, lanes 6 and 7), but not by GST alone (lane 2) or the other WWOX domains (lanes 3–5) examined. These results suggest that GSK3β interacts with WWOX by binding to its ADH domain.
Leucine 404 within the ADH domain is critical for WWOX associated with GSK3β. (a) WWOX constructs used in this study. Full-length WWOX, ww1, ww2, ww, ADH domain and mutant WWOX (L311A and L404A) were cloned into a GST-fusion protein expression vector. Two partial deletions of the WWOX ADH domain are indicated (Δ389 and Δ286). (b) Each GST-tagged WWOX construct was prepared and incubated with cell lysates in a binding buffer. Equivalent loading of the proteins was confirmed using Coomassie blue staining. Western blot analysis with anti-GSK3β antibodies was used to verify the interaction domain. (c) GST, WWOX and two WWOX-derived GST-fusion proteins (Δ389 and Δ286) were analysed in a GST pull-down assay. (d) GST pull-down assay with GST or GST-fused mutant WWOX (L311A and L404A). (e) SH-SY5Y cells were transfected with GFP, GFP–WWOX or GFP–WWOXL404A and harvested for immunoprecipitation with anti-GSK3β. Anti-GFP was used to detect GFP–WWOX. (f–h) Molecular modelling of the WWOX–GSK3β interaction. (f) An overview of the interaction of WWOX388−407 and GSK3β. (g) A close-up view of the interface between the GSK3β binding-pocket α-helix and WWOX388−407. The two binding partners are shown at a distance for clarity. (h) A close-up view of the interface between the GSK3β-binding pocket loop and WWOX388−407. The two binding partners are shown at a distance for clarity. Colour code: WWOX388−407, pink; GSK3β-binding pocket α-helix, red; GSK3β-binding pocket loop, green; GSK3β non-interacting regions, blue. Red dashed lines indicate electrostatic interactions, namely hydrogen bonds or salt bridges; green dashed lines indicate hydrophobic interactions
Additionally, the GST pull-down assay demonstrated that neither WWOX-Δ389 (1–389 a.a.) nor WWOX-Δ286 (1–286 a.a.) were able to interact with GSK3β (Figure 3c). This indicates that WWOX amino acids 388–407 are required for its interaction with GSK3β. Furthermore, bioinformatics analyses revealed that L404 was highly conserved in WWOX and other GSK3β-binding proteins (Figure 2a). In order to clarify whether L404 within the highly conserved GSK3β-binding motif, LXXRL, has an important role in GSK3β binding, a GST pull-down assay was performed using two WWOX mutants, L404A and L311A. As shown in Figure 3d, the mutation of L404A, but not L311A, completely abolishes the binding of WWOX to GSK3β. These results suggest that WWOX physically interacts with GSK3β in vitro through the L404-containing motif within the WWOX388−407 region. Finally, the co-immunoprecipitation experiment was performed again to compare wild-type (WT) WWOX with these mutants. As expected, ectopic GFP–WWOX can be co-immunoprecipitated by endogenous GSK3β, and this interaction was diminished when L404 mutated to alanine (Figure 3e, right panel). Similar results were found in a co-immunoprecipitation experiment using GFP antibodies against GFP-tagged WWOX proteins (Figure 3e, left panel).
To investigate the possibility that the L404 mutant is incorrectly folded, thus disrupting GSK3β–WWOX interaction, we conducted co-immunoprecipitation experiments using c-jun, a WWOX-binding protein. C-jun was immunoprecipitated by either WT or mutant WWOX–GFP, indicating that WWOX L404A is folded correctly.17 To understand how WWOX associates with GSK3β, we simulated the interaction in silico using molecular modelling. Figure 3f illustrates the interaction between GSK3β and WWOX388−407. The overview in Figure 3f shows that the motif portion of WWOX388−407 (highlighted in light pink and dark pink, respectively), especially L400, R403 and L404, are in close contact with a binding pocket α-helix (G262-L273, red) and loop (N285-H299, green) on GSK3β. Figures 3g and h and Supplementary Figure B show close-up views of WWOX388−407 interacting with the binding pocket α-helix and loop, respectively. As indicated in Figure 3g, the interactions between WWOX388−407 and the binding pocket α-helix are hydrophobic (indicated by red dashed lines) and electrostatic (indicated by green dashed lines). The van der Waals interactions included WWOX388−407 L400 with a number of consecutive hydrophobic residues, including V263, L266, V267 and I270; WWOX388−407 L404 with V267 and L270; and WWOX388−407 A399 with V263. A similar helix–helix ridge-groove hydrophobic interaction was also present in the GSK3β–GSKIPtide18 and GSK3β–AxinGID19 structures. Similarly, the contact between WWOX388−407 and the GSK3β-binding loop was a simple hydrophobic interaction between L400 and F293, and between L404 and I296. Collectively, these results revealed that the L404-containing motif located within WWOX388−407 is essential for the association of WWOX and GSK3β.
GSK3β kinase activity is negatively regulated by WWOX
We next investigated the functional effect of WWOX in regulating GSK3β activity. We performed an in vitro kinase assay to examine whether WWOX interferes with GSK3β-mediated Tau phosphorylation. GST-tagged WWOX protein (0.3 μg) and His-GSK3β protein (30 ng) were incubated in reaction buffer for 30 min before adding 0.5 μg His-Tau protein and a [γ-32P] ATP cocktail. The GSK3β-mediated Tau phosphorylation was examined by autoradiography and western blotting analysis. As shown in Figure 4a, GSK3β can phosphorylate Tau in vitro, and this phosphorylation is completely abolished in the presence of excess WWOX, but not GST or WWOX L404A. Moreover, we demonstrated that GSK3β-mediated Tau phosphorylation at S396 and S404 was reduced in a dose-dependent manner by GST–WWOX (Figure 4b). In the same experiment, we investigated the specificity of the effect of WWOX on GSK3β-mediated Tau phosphorylation by examining the phosphorylation of S422, which is phosphorylated by MKK4 kinase in vivo (Figure 4c). SH-SY5Y cells were transiently transfected with GFP and GFP–WWOX for 12 h, and the cell lysate was subjected to western blotting. Ectopically expressed WWOX significantly inhibited Tau phosphorylation at S404 and S396 but not S422. However, this inhibition was diminished when WWOX L404A was used. These results again confirm our finding that the binding of WWOX to GSK3β is required for its inhibition of GSK3β-mediated Tau phosphorylation. The phosphorylation of Tau S422 is not affected by WWOX (Figure 4c), suggesting that the inhibition of GSK3β through the binding of WWOX is highly specific, at least in SH-SY5Y cells. Moreover, we examined the effect of WWOX on the activity of GSK3β toward the peptide substrate GS-1, which is derived from glycogen synthase. Consistent with our observations of Tau phosphorylation, WT but not mutant WWOX inhibits GSK3β phosphorylation of GS-1 (Supplementary Figure C).
WWOX suppresses Tau phosphorylation. (a) GST, GST–WWOX or GST–WWOX L404A (0.2 μg) were incubated with 30 ng His-GSK3β for 30 min in a reaction buffer and incubated with 0.5 μg Tau protein, ATP cocktail, and 10 Ci of [γ-32P] ATP for 10 min at 20 °C. The reaction was stopped by adding 2 × sample buffer and heating at 100 °C for 10 min. This was followed by SDS-PAGE, and phosphorylation signals were evaluated using autoradiography. (b) WWOX (1, 3 and 10 μg) inhibited Tau phosphorylation by GSK3β in a dose-dependent manner. GST–WWOX was pre-incubated with His-GSK3β for 30 min in a reaction buffer including optimised quantities of ATP and Tau protein. The reactions were then subjected to western blot analysis of Tau, pTauS396 and S404. (c) SH-SY5Y cells were transiently transfected with GFP, GFP–WWOX or GFP–WWOX L404A. Cells were lysed, and a western blot analysis was carried out using antibodies against pTauS396, pTauS404, pTauS422, Tau and actin. Actin expression was evaluated to verify equal loading, and GFP expression was evaluated to confirm the protein expression level. (d) A scrambled peptide and WWOXtide388−407 were each incubated with 30 ng His-GSK3β, ATP-cocktail, and 10 Ci of [γ-32P] ATP for 10 min at 20 °C. The reaction was stopped by adding 2 × sample buffer and was followed by SDS-PAGE. Phosphorylation was detected with autoradiography
We subsequently performed a competitive inhibition assay to further support our conclusion using WWOXtide388−407, a WWOX-derived peptide, in the in vitro kinase assay experiment. WWOXtide388−407, but not the control scrambled peptide, inhibited GSK3β activity and reduced Tau phosphorylation, as shown by autoradiography (Figure 4d). Collectively, our results reveal that WWOX negatively regulates GSK3β kinase activity on the neuron-specific microtubule-binding protein Tau in SH-SY5Y cells.
WWOX promotes neuronal cell differentiation through the inhibition of GSK3β
Researchers have suggested that GSK3β has an important role in cell differentiation. We therefore investigated whether GSK3β inactivation is involved in RA-stimulated neuronal cell differentiation. SH-SY5Y cells were transiently transfected with a GFP control or GSK3β (WT, S9A or kinase dead (KD), or R96A) for 24 h, and the neurite outgrowth of SH-SY5Y cells, indicating neuronal cell differentiation, was analysed. Transfection with GFP–GSK3β WT and S9A notably decreased SH-SY5Y cell differentiation, whereas KD and R96A did not affect SH-SY5Y cell differentiation compared with the GFP control (Figures 5a and b). GSK3β small interference RNA (siRNA) was used to knock down GSK3β protein expression to confirm the crucial role of GSK3β inactivation in RA-mediated axon growth. SH-SY5Y cells were transfected with GSK3β siRNA, followed by treatment with 10 μM RA for 1 to 4 days. Compared with the control scrambled-RNAi group, the GSK3β protein-knockdown group showed significantly increased neurite outgrowth percentage beginning on the second day of RA treatment (Figures 5c and d). Thus, our results showed that GSK3β could negatively regulate neurite outgrowth in RA-stimulated neuronal cells.
WWOX regulates neuronal differentiation through its interaction with GSK3β. (a) SH-SY5Y cells were transfected with GFP, GFP–GSK3β WT, S9A, K8586MI and R96A for 24 h. The cells were then treated with 10 μM RA for 24 h. Cells with neurites longer than twice the length of the cell body were counted. Data are presented as the mean±S.E. from three independent experiments. **P<0.01 compared with the GFP control. (b) Western blot analysis confirmed the expression of the GFP-fusion protein by the anti-GFP antibody. (c) SH-SY5Y cells were transfected with pre-designed siRNA targeting GSK3β for 24 h and treated with 10 μM RA for 1 to 4 days. Cells with neurites longer than twice the length of the cell body were counted. Bright-field results are shown to indicate post-differentiation status at 24 h. Data are presented as the mean±S.E. from three independent experiments. *P<0.05 compared with mock control. (d) Cells were treated with siRNA and lysed in 2 × sample buffer. Western blot analysis was carried out using antibodies against GSK3β. The blot was stained for actin to verify equal loading. (e) Tau protein was incubated with GST, GST–WWOX or GST–WWOX L404A in the presence of GSK3β for the microtubule assembly assay. The reaction mixture also contained tubulin protein, GTP, and reaction buffer. The reaction mixture was incubated at 37 °C in a thermostatic spectrophotometer, and the change in reflectance over time was measured at 350 nm. (f) SH-SY5Y cells were transfected with empty vector, GFP–WWOX or GFP–WWOXL404A, as indicated. After transfection, cells were washed, and the medium was replaced with 1% serum and treated with 10 μM RA. The photos were taken on day 1. (g) The percentage of differentiated cells with neurites longer than twice the length of the cell body was calculated. The data are presented as the mean±S.E. from three independent experiments. *P<0.05 compared with the mock-treated control
Microtubule polymerisation is indispensable in neurons because neurite growth is severely disrupted when polymerisation is inhibited, indicating that a constant flux of microtubules is required for axonal growth. Furthermore, evidence shows that the phosphorylation of Tau by GSK3β affects the ability of Tau to promote microtubule formation.20, 21, 22 This evidence, in addition to the results of this study, suggests that WWOX may promote neuronal cell differentiation by inhibiting GSK3β-mediated Tau phosphorylation. We therefore used a light-scattering assay to clarify how WWOX and GSK3β affect Tau-mediated microtubule assembly. As shown in Figure 5e, we measured the effect of GSK3β on the turbidimetric time course of microtubule assembly. GSK3β diminished the microtubule assembly, as reflected by decreased turbidity. However, the new equilibrium reached a higher turbidity when treated with WT WWOX and GSK3β than with GSK3β alone. Interestingly, the GSK3β-binding deficient WWOX, WWOX L404A, could not restore the microtubule assembly activity. In conclusion, our data demonstrated that WWOX could restore the ability of Tau to promote microtubule formation by inhibiting GSK3β activity.
WWOX L404A, which is deficient in binding to GSK3β (Figure 3) and was incapable of restoring Tau activity on microtubule formation (Figure 5e), should fail to stimulate neuronal cell differentiation. To confirm this prediction, the SH-SY5Y cell differentiation promoted by WWOX L404A was examined and compared with that of WT WWOX. WT WWOX and WWOX L404A were separately overexpressed in SH-SY5Y in the presence of RA treatment. WWOX significantly increased the percentage of differentiated SH-SY5Y cells. However, this increase was abolished when L404 was mutated to alanine. These results strongly suggest that WWOX promotes neuronal differentiation in SH-SY5Y cells by inhibiting the GSK3β-mediated phosphorylation of the microtubule-binding protein Tau (Figures 5f and g).
To demonstrate that Tau is indeed the ultimate effector of WWOX/GSK3β-dependent neuronal cell differentiation, we performed double manipulation of WWOX and GSK3β, as well as the delivering of siRNA to knock down Tau and GSK3β, respectively, and then conducted RA-induced neurite outgrowth experiments. Figures 6a and b (lanes 1–3) show that the neurite outgrowth stimulated by RA was abolished when Tau was knocked down. Western blotting (Figure 6b) confirmed protein overexpression or knockdown after transient transfection of the WWOX-expressing vector or Tau and GSK3β siRNAs. Neuronal differentiation increased to 44% in the WWOX overexpression group comparing with 18% of RA-control cells group (lane 6). This increase was completely abolished when Tau was knocked down in a double manipulation experiment (lane 5). Similar results were exhibited in a GSK3β and Tau double manipulation experiment, which showed that neuronal cell differentiation of the GSK3β knockdown group increased approximately 25% more than the RA-control (lane 7), which was completely abolished when Tau was knocked down (lane 4). Neither WWOX overexpression nor GSK3β knockdown promoted neurite outgrowth in the Tau knockdown condition, indicating that Tau is the effector of both WWOX and GSK3β (Figures 6a and b). Interestingly, co-transfection of the WWOX plasmid and GSK3β siRNA followed by RA treatment did not show an additive or synergistic effect compared with WWOX overexpression alone or GSK3β knockdown alone (Figures 6a and b, lane 8). WWOX did not further promote neurite outgrowth in the presence of GSK3β siRNA, consistent with the hypothesis that WWOX promotes neuronal differentiation through GSK3β inhibition. Collectively, these results connect WWOX, GSK3β and Tau in an exclusive, direct manner and show that WWOX promotes neuronal SH-SY5Y cell differentiation through inhibiting the GSK3β-mediated phosphorylation of the microtubule-binding protein, Tau.
Tau is indispensable for WWOX regulation of neuronal differentiation. (a) SH-SY5Y cells were transfected with Tau siRNA, GSK3β siRNA and WWOX together or separately for 24 h. They were then treated with 10 μM RA for another 24 h. The photos were taken on day 1. (b) The percentage of differentiated cells with neurites longer than twice the length of the cell body was calculated. (c) Cells treated with Tau and GSK3β siRNA and WWOX plasmid were lysed in 2 × sample buffer, and a western blot analysis was carried out using antibodies against Tau, WWOX and GSK3β. (d) Our proposed model, showing that WWOX abolishes GSK3β kinase activity and therefore restores the ability of Tau to promote microtubule assembly
Discussion
In this study, we found two GSK3β consensus-binding sites in the WWOX amino-acid sequence. Furthermore, our results show that WWOX physically interacts with GSK3β only through the WWOX388−407 region, which is an L404-containing motif. Some reports suggest that L400 is also important for WWOX to associate with GSK3β. Smalley et al.19 demonstrated that L392 and L396 are essential for the interaction between Axin1 and GSK3β. Interestingly, the L392 and L396 of Axin1 are related to L400 and L404, respectively. These results show that WWOX interacts with GSK3β through a structure and sequence similar to that of Axin381−405. Furthermore, researchers have suggested that GSK3β has an important role in multiple cellular processes, including protein synthesis, glycogen metabolism, proliferation, microtubule dynamics and cell differentiation.23 WWOX may help to control certain types of cellular processes through the inhibition of GSK3β.
WWOX expression in the developing brain is low during the early embryonic stage when organogenesis is most rapid; however, moderate-to-high WWOX immunoreactivity is detected in the brain and spinal cord during the middle and late fetal stages.12 This strongly suggests that WWOX may have an important role in neuron differentiation. In this study, highly differentiated SH-SY5Y cells were correlated with high WWOX protein expression and low Tau S396 and S404 protein phosphorylation. This indicates that SH-SY5Y cell differentiation requires WWOX protein expression and lower pTau S396 and pTau S404 levels, possibly caused by the inhibition of GSK3β. Intriguingly, Sze's group demonstrated that the downregulation of WWOX induces Tau phosphorylation. They also showed that WWOX was downregulated in AD patients.2 Furthermore, the level of active GSK3β is reportedly increased in AD.24 Based on our results, we speculate that the upregulation of GSK3β, and therefore Tau phosphorylation, in AD may due to the downregulation of WWOX.
Chen et al.12 reported that WWOX was differentially expressed during various stages of brain development in mice, indicating its potential role in promoting neuronal differentiation and maturation. They also used a yeast two-hybrid assay to demonstrate that WWOX associates with Tau and showed that the downregulation of WWOX may induce Tau phosphorylation in vitro.2 However, the molecular mechanism by which WWOX facilitates neural differentiation has yet to be revealed. Here, we were not able to detect a Tau–WWOX complex in our co-immunoprecipitation experiment from SH-SY5Y cells (Supplementary Figure A); the inconsistency in results may due to the different expression systems used. However, our results connect WWOX, GSK3β and Tau in an exclusive, direct manner and describe a novel mechanism by which WWOX promotes neuronal SH-SY5Y cell differentiation by inhibiting the GSK3β-mediated phosphorylation of the microtubule-binding protein, Tau.
The extension of immature neurites requires the assembly of a stable microtubule infrastructure. Microtubule polymerisation and stability are regulated by a number of microtubule-associated proteins, including Tau. GSK3β regulates Tau through phosphorylation; hyperphosphorylated Tau has a decreased affinity for microtubules and disrupts microtubule stability. This study shows that the knockdown of GSK3β expression through RNAi decreases Tau phosphorylation and increases the occurrence of SH-SY5Y cell differentiation. Additionally, researchers have reported that the inactivation of GSK3β results in the dephosphorylation of the GSK3β downstream target, CRMP-2, which leads to enhanced microtubule polymerisation and axon growth.11 Therefore, neurite outgrowth requires microtubule assembly and the inhibition of GSK3β activity. However, a number of previous studies have reported that the activation of GSK3β enhanced neurite growth rates in PC12, NT2 or SH-SY5Y cells.25, 26, 27 However, our microtubule assembly assay clearly showed that WWOX restores the microtubule assembly-promoting activity of Tau that is repressed by GSK3β. Ahmad et al.21 reported that the inhibition of microtubule polymerisation compromises axonal growth. We observed highly phosphorylated Tau in undifferentiated SH-SY5Y cells. Reduced Tau phosphorylation also correlated strongly with WWOX expression in highly differentiated SH-SY5Y cells. These results provide evidence that WWOX has an important role in inhibiting GSK3β activity and suppressing Tau hyperphosphorylation.
In conclusion, our study demonstrates that WWOX physically interacts with GSK3β through its ADH/SDR domain and decreases Tau protein hyperphosphorylation at S404 and S396, both in vivo and in vitro. Furthermore, we showed that an increased WWOX protein level is correlated with decreased pTau S396 and S404 levels in highly differentiated SH-SY5Y cells. The microtubule assembly will eventually be promoted by Tau when GSK3β is inhibited by WWOX during RA-stimulated neuronal cell differentiation. We thus present a novel mechanism for the regulation of neuronal differentiation by WWOX.
Materials and Methods
Cell culture, transfection and treatments
Human neuroblastoma SH-SY5Y cells were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal calf serum and antibiotics (100 mg/ml streptomycin and 100 IU/ml penicillin; Life Technologies, Inc., Gaithersburg, MD, USA). The cells were grown to subconfluence and then treated with 10 μM RA for 1 to 5 days. The information of siRNA used in this study was provided in Supplementary Table 1. For efficient transfection, Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer's instructions.
Plasmid construction
A full-length WWOX (human WOX1) construct was subcloned into the following expression vectors: pEGFP-C1-CPO, pGEX-KG and pcDNA3.1-HA-CPO. The ADH domain fragment was generated by PCR with pfu polymerase (Promega, Madison, WI, USA). The PCR product was subcloned into the pGEX-KG, pEGFP-C1-CPO and pcDNA3.1-HA-CPO plasmids for sequence verification and further cloning.
WWOX mutants (L311A and L404A) were prepared using site-directed mutagenesis and a two-step PCR technique. After successful cloning, the WWOX cDNA insert sequences were further confirmed through DNA sequencing.
Western blotting
Cells were disrupted in 2X sample buffer (0.1 M Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 2% β-mercaptoethanol), boiled for 10 min, centrifuged, placed on ice for several minutes, stored at −80 °C and then separated using 10–12.5% SDS-polyacrylamide gel electrophoresis (PAGE). Proteins were then electrotransferred onto PVDF transfer membranes (Millipore, Lincoln Park, NJ, USA). The membranes were blocked with 5% nonfat milk in TBST buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Tween 20) containing 5% bovine serum albumin at 4 °C for 2 h or overnight, and incubated for 2 h at room temperature with one of the following primary antibodies: monoclonal anti-GFP (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GSK3β (BD Transduction Laboratories, Lexington, KY, USA), monoclonal anti-actin (Sigma, St. Louis, MO, USA), Tau (Millipore), rabbit anti-pTau S396 (Origene, Rockville, MD, USA), rabbit anti-pTau S404 (Millipore) or rabbit anti-pTauS422 (Abcam, London, UK). The membranes were washed with TBST six times every 10 min. Immunoreactive proteins were detected using horseradish peroxidase-conjugated secondary antibodies and then the membrane was washed with TBST six times every 10 min during the detection process. The protein signal was detected by an enhanced chemiluminescence system (ECL Plus; Perkin Elmer Life and Analytical Sciences, Inc., Waltham, MA, USA).
Immunoprecipitation and GST pull-down assays
For immunoprecipitation, SH-SY5Y cells were washed twice with phosphate-buffered saline (PBS) at the indicated times and lysed in ice-cold RIPA buffer (100 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Tween 20, 0.1% Triton X-100, 1 mM DTT, 50 μg/ml AEBSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 mM NaF and 1 mM Na3VO4). Anti-WWOX antibodies were incubated with Protein G-Sepharose beads for 1 h at 4 °C, and the pretreated cell lysates were added to the Protein G-Sepharose beads for 2 h at 4 °C. The immune complexes were then washed three times with a RIPA buffer, eluted by boiling in a 2X SDS sample buffer, and subjected to western blotting analysis. For GST pull-down assays, GST or GST–WWOX proteins expressed in BL21 (DE3) were adsorbed to glutathione-agarose beads (Sigma) for 1 h after three washes with PBS. The beads were then washed three times with the same buffer. Sample buffer was added, and the beads were boiled and subjected to western blot analysis.
Immunofluorescence assays
SH-SY5Y cells co-transfected with pcDNA3.1–HA–WWOX and GFP–GSK3β were grown on coverslips, washed in PBS, fixed with 3.7% paraformaldehyde in PBS, and permeabilised by incubation in 10% Triton X-100. Following permeabilisation, the cells were washed with PBS and incubated with a mouse anti-HA monoclonal antibody (Santa Cruz Biotechnology) at 4 °C overnight. The cells were washed again and then incubated with goat anti-mouse IgG rhodamine (Santa Cruz Biotechnology). The cells were washed again and subsequently stained with DAPI. In each instance, the cells were washed in PBS. Fluorescently labelled protein localisation was visualised using a Zeiss laser confocal microscope (LSM510; Zeiss Inc, Oberkochen, Germany).
In vitro kinase assay
His-tagged human Tau and His-tagged human GSK3β were expressed in E. coli BL21 and purified by means of affinity chromatography. The purity of the recombinant proteins was verified by SDS-PAGE. The purified recombinant GSK3β protein was pre-incubated with WT or mutant GST–WWOX and 25 μg/ml His-Tau for 30 min, and the kinase reaction was initiated by the addition of an equal volume of 2X reaction buffer (100 mM HEPES, pH 7.4, 1 mM DTT and 10% glycerol) and ATP cocktail (20 mM ATP and 1 mM MgCl2). After incubating the mixtures at 30 °C for up to 20 min, the reaction was stopped by adding 2 × sample buffer and heating at 100 °C for 10 min. The proteins were further separated using SDS-PAGE. Results were detected using autoradiography or western blotting.
Molecular dynamics simulation
A molecular dynamics (MDs) simulation was employed to predict the binding interaction between GSK3β and WWOX388−407. As the structure of WWOX is unknown, the GOR IV Secondary Structure Prediction Method28 was used to predict the structure of the WWOX388−407 peptide. Accordingly, the structure of the GSK3β–AxinGID complex (PDB entry code: 1O9U) was used as a template to create an initial structure for the GSK3β–WWOX388−407 complex. The structure of AxinGID was extracted from the complex, and the amino acids were modified to generate WWOX388−407. The resulting WWOX388−407 peptide was then subjected to energy minimisation in which the backbone was constrained and the side chains were allowed to achieve their proper orientations using 5000 steps of the steepest descent algorithm and 5000 steps of the conjugate gradient algorithm. The X-ray structure of GSK3β–AxinGID served as a guide for orienting WWOX388−407 in the GSK3β-binding pocket, which was composed of an α-helix, G262-L273, and an extended loop from N285 to H299. Energy minimisation was applied to eliminate any residual geometrical strain caused by placing WWOX388−407 in the GSK3β-binding pocket. Energy minimisation was achieved by performing 5000 steps of the steepest descent algorithm and 5000 steps of the conjugate gradient algorithm.
The MD simulations in this study were performed according to the standard protocol, which consists of gradual heating, equilibration and production procedures in an isothermal isobaric ensemble (NPT, P=1 a.t.m. and T=300 K) MD. A minimised, solvated system was used as the initial structure for subsequent MD simulations. In the 150 p.s. heating procedure, the system was gradually heated from 50 to 300 K at a rate of 2.4 K/1 p.s., followed by constant equilibration at 300 K for 150 p.s. After the equilibration procedure, the system underwent a 2 n.s. production procedure for conformation collection. The time step was set at 1 f.s. A snapshot was taken every 5 p.s. to record the conformation trajectory during MD production. A 12- Å cutoff was applied to treat non-bonding interactions, such as short-range electrostatic and van der Waals interactions. The particle-mesh-Ewald (PME) method was applied to treat long-range electrostatic interactions. All simulations were performed using Accelrys' Discovery Studio software with a CHARMM force field. The SHAKE algorithm was used to constrain all bonds containing hydrogen bonds to their equilibrium lengths.
Microtubule assembly assay
Microtubule assembly assays were performed by incubating tubulin (0.2 mg/μl) (Cytoskeleton; Denver, CO, USA) in cuvettes at 37 °C in a thermostatic spectrophotometer and measuring the turbidity change at 350 nm over time. To examine the effects of WWOX and GSK3β on tubulin polymerisation, the GSK3β was pre-incubated with Tau in the presence of WWOX for 30 min, and polymerisation was then initiated by the addition of 1 mM GTP and followed by a light-scattering assay.
Abbreviations
- RA:
-
retinoic acid
- WWOX:
-
WW domain-containing oxidoreductase
- GSK3β:
-
glycogen synthase kinase 3β
- ADH/SDR:
-
short-chain alcohol dehydrogenase/reductase
- GSKIP:
-
glycogen synthase kinase 3β interaction protein
- CRMP-2:
-
collapsin response mediator protein 2
- MD:
-
molecular dynamic
- GST:
-
glutathione S-transferase
- GFP:
-
green fluorescent protein
- AD:
-
Alzheimer's disease
References
Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D et al. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA 2001; 98: 11417–11422.
Sze CI, Su M, Pugazhenthi S, Jambal P, Hsu LJ, Heath J et al. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer's disease. J Biol Chem 2004; 279: 30498–30506.
Smith DI, McAvoy S, Zhu Y, Perez DS . Large common fragile site genes and cancer. Semin Cancer Biol 2007; 17: 31–41.
Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J et al. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 2009; 69: 4835–4842.
Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM . Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64: 8256–8261.
Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM . WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22.
Doble BW, Woodgett JR . GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 2003; 116 (Part 7): 1175–1186.
Hedgepeth CM, Deardorff MA, Rankin K, Klein PS . Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin. Mol Cell Biol 1999; 19: 7147–7157.
Chou HY, Howng SL, Cheng TS, Hsiao YL, Lieu AS, Loh JK et al. GSKIP is homologous to the Axin GSK3beta interaction domain and functions as a negative regulator of GSK3beta. Biochemistry 2006; 45: 11379–11389.
Zhou F, Zhang L, Wang A, Song B, Gong K, Zhang L et al. The association of GSK3 beta with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J Biol Chem 2008; 283: 14506–14515.
Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K . GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 2005; 120: 137–149.
Chen ST, Chuang JI, Wang JP, Tsai MS, Li H, Chang NS . Expression of WW domain-containing oxidoreductase WOX1 in the developing murine nervous system. Neuroscience 2004; 124: 831–839.
Maden M . Retinoid signalling in the development of the central nervous system. Nat Rev 2002; 3: 843–853.
Clagett-Dame M, McNeill EM, Muley PD . Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J Neurobiol 2006; 66: 739–756.
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 2000; 75: 991–1003.
Hernandez F, Lucas JJ, Cuadros R, Avila J . GSK-3 dependent phosphoepitopes recognized by PHF-1 and AT-8 antibodies are present in different tau isoforms. Neurobiol Aging 2003; 24: 1087–1094.
Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM et al. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66: 11585–11589.
Tang XN, Lo CW, Chuang YC, Chen CT, Sun YC, Hong YR et al. Prediction of the binding mode between GSK3β and a peptide derived from GSKIP using molecular dynamics simulation. Biopolymers 2011; 95: 461–471.
Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V et al. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J 2003; 22: 494–501.
Utton MA, Vandecandelaere A, Wagner U, Reynolds CH, Gibb GM, Miller CC et al. Phosphorylation of tau by glycogen synthase kinase 3beta affects the ability of tau to promote microtubule self-assembly. Biochem J 1997; 323 (Part 3): 741–747.
Ahmad FJ, Joshi HC, Centonze VE, Baas PW . Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron 1994; 12: 271–280.
Wagner U, Utton M, Gallo JM, Miller CC . Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 1996; 109 (Part 6): 1537–1543.
Jope RS, Yuskaitis CJ, Beurel E . Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007; 32: 577–595.
Leroy K, Yilmaz Z, Brion JP . Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol 2007; 33: 43–55.
Goold RG, Gordon-Weeks PR . The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol Cell Neurosci 2005; 28: 524–534.
Gompel M, Soulie C, Ceballos-Picot I, Meijer L . Expression and activity of cyclin-dependent kinases and glycogen synthase kinase-3 during NT2 neuronal differentiation. Neuro-Signals 2004; 13: 134–143.
Castano Z, Gordon-Weeks PR, Kypta RM . The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth. J Neurochem 2011; 113: 117–130.
Garnier J, Gibrat JF, Robson B . GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 1996; 266: 540–553.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Deshmukh
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, HY., Juo, LI., Lin, YT. et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ 19, 1049–1059 (2012). https://doi.org/10.1038/cdd.2011.188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2011.188
Keywords
This article is cited by
-
Wwox deficiency leads to neurodevelopmental and degenerative neuropathies and glycogen synthase kinase 3β-mediated epileptic seizure activity in mice
Acta Neuropathologica Communications (2020)
-
Alzheimer’s Disease Genetics: Review of Novel Loci Associated with Disease
Current Genetic Medicine Reports (2020)
-
A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency
Cell Death & Disease (2015)
-
WWOX dysfunction induces sequential aggregation of TRAPPC6AΔ, TIAF1, tau and amyloid β, and causes apoptosis
Cell Death Discovery (2015)
-
Role of WWOX and NF-κB in lung cancer progression
Translational Respiratory Medicine (2013)