Abstract
After genotoxic stress, normal cells trigger DNA repair or, if unable to repair, undergo apoptosis to eradicate the cells that bear the risk of becoming tumorigenic. Here we show that repression of the transcription factor, activating transcription factor 3 (ATF3), after ultraviolet (UV)-mediated genotoxic stress impairs the DNA repair process. We provide evidence that ATF3 directly regulates the proliferating cell nuclear antigen (PCNA)-associated factor KIAA0101/p15PAF. We further show that the expressions of ATF3 and p15PAF is sufficient to trigger the DNA repair machinery, and that attenuation of their expression alters DNA repair mechanisms. We show that the expression of p15PAF compensates for a lack of ATF3 expression, thereby constituting a major effector of ATF3 in the DNA repair process. In addition, we provide evidence that p15PAF expression is required for the correct function of PCNA during DNA repair, as prevention of their interaction significantly alters DNA repair mechanisms. Finally, defective DNA repair, because of the downregulation of p15PAF expression, rendered the cells more sensitive to UV-induced cell death. Therefore, our results suggest ATF3 and p15PAF as novel gatekeepers of genomic integrity after UV exposure.
Similar content being viewed by others
Main
Environmental genotoxic stress is one of the major causes of genomic instability. During such a stress, the maintenance of genomic integrity is of crucial importance to allow correct cellular function. The nucleotide excision repair (NER) machinery is responsible for recognition and incision of DNA lesions caused by ultraviolet (UV) irradiation and chemical agents.1 Proliferating cell nuclear antigen (PCNA) is responsible for recruitment of Polδ and, therefore, mediates DNA re-synthesis within the site of the lesion.1 The importance of such machinery is underscored by the numerous human syndromes, such as xeroderma pigmentosum (XP) and trichothiodystrophy (TTD), caused by the mutation of genes involved in DNA repair. Such mutations compromise genomic integrity, and XP and TTD patients are highly photosensitive and prone to develop skin tumors of keratinocyte origin, such as basal and squamous cell carcinoma. Severe defects in the DNA repair mechanism thus impair the apoptotic pathway responsible for destruction of cells that bear the risk of becoming tumorigenic. The correct DNA damage response is therefore of great importance in the maintenance of a UV-inducible anticancer barrier that elicits growth arrest, repair or cell death.
Activating transcription factor 3 (ATF3) is a member of the ATF/CREB subfamily of the basic region leucine zipper (bZIP) family and may be strongly induced in a variety of tissues by different stress signals.2 Several pathways are responsible for ATF3 induction, such as ATM and Nibrin 1, JNK and NF-κB, in a p53-dependent or -independent manner.3, 4, 5 Induction of ATF3 often correlates with cellular damage, suggesting an important role during the cellular stress response.6 We have recently reported that the alteration of Egr-1 expression during UV-mediated genotoxic stress has serious consequences for proapoptotic gene expression, thus leading to an enhanced cell survival.7, 8 Similar to Egr-1, ATF3 is greatly induced after UV irradiation and acts as a pro-apoptotic regulator9, 10, 11 in cells destined to die. This finding raises the issue about DNA damage status of the surviving cells. Do the cells survive because they have efficiently repaired their DNA or do they survive while accumulating DNA damage?
This study aims to determine whether ATF3 is involved in the DNA repair pathway. We provide evidence that ATF3 directly regulates the PCNA-associated factor KIAA0101/p15PAF and further show that ATF3 and p15PAF gene expressions are necessary for the DNA repair process. In addition, we provide evidence that p15PAF expression is required for correct PCNA function, as prevention of their interaction alters DNA repair mechanisms.
Results
ATF3 directly regulates the PCNA-associated factor KIAA0101/p15PAF
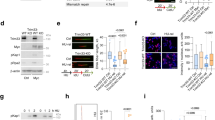
To characterize ATF3 function after UV stress, knockdown experiments were performed, using a previously described siRNA (siATF3)12. The efficiency of this siRNA in HaCaT and HeLa cells has been shown in an earlier study.10 The transcriptome of HeLa cells rendered deficient in ATF3 expression was compared with that of control nonrelevant siRNA (siLuc)-transfected cells, 2.5 h after UVC irradiation. Among the candidates that were found to be upregulated by ATF3 (i.e., downregulated by the knockdown of ATF3), we identified the PCNA-associated factor KIAA0101/p15PAF. A quantitative real-time PCR (QPCR) analysis further confirmed the findings from our microarray study (Figure 1) and showed a clear correlation between the inhibition of ATF3 and the reduction of p15PAF expression. A time course of the expressions of ATF3 and p15PAF mRNA was performed in UVC-irradiated wild type (WT) and ATF3 (−/−) mouse embryonic fibroblast (MEF) cells (Figure 2a, right panel) as well as in HaCaT keratinocyte cells rendered deficient in ATF3 expression by siRNA treatment (Figure 2a, left panel). These experiments show that the p15PAF mRNA expression was induced by UV in the presence of ATF3 in each cell line, whereas no significant induction was observed in both siATF3-treated HaCaT and ATF3 (−/−) MEF cells. Transfection of these cells with a construct encoding human ATF3 under basal or UVC (5 J/m2)-irradiated conditions released p15PAF expression (Figure 2b). Accordingly, an increased expression of ATF3 in HeLa cells lead to a dose-dependent induction of p15PAF (Figure 2c). Furthermore, the loss of p15PAF mRNA leads to a corresponding effect at the protein level, as the knock down of ATF3 drastically eliminated the p15PAF protein expression (Figure 2d). Similar results were obtained using a different siRNA specific to ATF3 (siATF3(2)) (Supplementary Figure 1a). We have further assessed whether ATF3 acts through direct binding to the regulatory sequences of the p15PAF gene promoter. Chromatin immunoprecipitation (ChIP) assays were, therefore, performed on HaCaT cells using a specific antibody raised against ATF3 and primers located within the proximal promoter of p15PAF gene. Significant PCR amplification was observed only for UVC-treated chromatin extracts immunoprecipitated with anti-ATF3 (Figure 2e). No amplification was observed with the control serum (nonimmune serum, or an immune but nonrelevant serum), the use of primers (P3) located at the boundary of a nonrelevant sequence or the nonirradiated extracts. These results clearly show the direct binding of ATF3 on the p15PAF proximal promoter. Taken together, our results show the major role of ATF3 in the regulation of p15PAF upon UV-mediated genotoxic stress.
A microarray analysis reveals p15PAF as a transcriptional target of ATF3. HeLa cells were transfected with siATF3 and siLuc siRNA as control. At 24 h after transfection, the cells were UV-irradiated (40 J/m2), and total RNA was extracted 2 h after UV stress. The samples were then co-hybridized to a pan genomic microarray. p15PAF and ATF3 expressions were detected by microarray (gray) and confirmed by real-time PCR (white). Hybridization and RT-PCR were performed in duplicate
ATF3 directly regulates p15PAF expression. (a) HaCaT cells were transfected either with siATF3 or siLuc and UVC-irradiated (5 J/m2) 24 h after transfection (left panel). The expression of p15PAF mRNA was determined by QPCR at each indicated time point (right panel). A similar time course experiment was performed using WT and ATF3 (−/−) MEF cells. These experiments were performed thrice in duplicate. (b) ATF3 (−/−) MEF cells were transfected with a construct encoding ATF3 protein and either left untreated or irradiated with 5 J/m2 UVC. Endogenous murine p15PAF expression was detected by QPCR. (c) Increasing amount of a construct encoding ATF3 protein was transfected into HaCaT cells. The expressions of ATF3 and p15PAF were detected by QPCR. QPCR experiments have been performed three independent times in duplicate. (d) At 2 h after UV irradiation, nuclear extracts were prepared from HaCaT cells transfected with siLuc or siATF3 and subjected to western blot analysis. Ctl corresponds to the ERK2 protein expression. (e) Chromatin immunoprecipitation experiment (ChIP). HaCaT cells were UVC-irradiated (UVC) or not (CTL), the chromatin extracts were immunoprecipitated with specific antibodies to ATF3 or nonimmune control serum (NI) or nonrelevant control anti-snap serum. The detection of p15PAF-captured promoter fragment was performed by PCR using two different primer pairs (P1 and P2). Primers (P3) located at the boundary of a nonrelevant sequence were used as a negative control. DNA input corresponds to a control genomic DNA. Primer sequences are provided in the Materials and Methods section
ATF3 and p15PAF are required for DNA repair
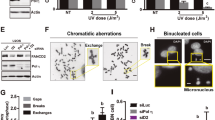
Exposure to UV radiation induces DNA alterations such as cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts. We have compared p15PAF and ATF3 protein expression profiles with the presence of CPDs. CPDs were still strongly detectable at 2 h after UV irradiation, but disappeared rapidly at 6 h. Cell recovery was almost complete 24 h after irradiation. Interestingly, ATF3 and p15PAF expressions peaked at 2 h after irradiation, suggesting a coordinated regulation with the presence of CPDs (Figure 3). To assess the direct contribution of both genes in the DNA repair process, HaCaT cells were rendered deficient by the transfection of siATF3 and sip15PAF,13 or siLuc as a control, 24 h after transfection, DNA damage was induced by irradiation with a single low dose of UVC (5 J/m2). After 24 h of recovery, the presence of CPD-positive cells was assessed. Excellent knockdown efficiencies of both siATF3 and sip15 were revealed by immunofluorescence and western blot experiments 2 h after irradiation (Figure 4a and b). Although almost no CPDs were detected when ATF3 and p15PAF were expressed (siLuc-transfected cells), a strong CPD staining was detected in cells lacking either protein (Figure 4a). Indeed, more than 70% of cells deficient for ATF3 or p15PAF are CPD-positive (Figure 4c). Similar results were obtained with an additional siRNA specific to ATF3 (Supplementary Figure 1b). To further confirm our results, DNA damage was analyzed in the same experimental setting by a PCR stop assay, 2 and 24 h after irradiation. This method is based on the inhibition of PCR amplification by CPDs. It has been shown that the DNA damage level determined by PCR amplification within a housekeeping gene reflects the global genomic damage level.14 A significant decrease in the amplification signal 2 h after irradiation showed the expected accumulation of damage (Figure 4d). After 24 h of recovery time, the PCR signal in the siLuc control condition increased nearly to the level observed in the nonirradiated sample, whereas only 11–12% increase was observed in the siATF3- and sip15PAF-treated samples (Figure 4d). Taken together, our results clearly show the requirement for ATF3 and p15PAF for optimal DNA repair upon UV stress. Interestingly, we have shown earlier the contribution of ATF3 to the mediation of UV-induced cell death, through the control of Hif2a expression.10 Therefore, ATF3 contributes to two important physiological events (DNA repair and death) triggered by UV irradiation. To elucidate how these two different physiological functions are orchestrated upon UV stress, we first analyzed Hif2a and p15PAF expressions after a dose response of UV (5, 20 and 40 J/m2). As expected, both genes are induced as low as 5 J/m2 of UV exposure. However, although p15PAF expression remained identical for each UV dose tested, Hif2a is induced in a UV dose-dependent manner and displayed highest expression levels at 40 J/m2 (Figure 5a). We have further assessed the effects of ATF3 and p15PAF knockdown on cell survival following increasing levels of UV exposure (5–80 J/m2). To achieve knock down, cells were transfected with appropriate siRNAs (siATF3, sip15PAF) or siLuc as a control. As expected, an increased cell survival was observed in cells deficient for ATF3. Interestingly, although p15PAF is a direct ATF3 target gene, its knock down produced an opposite effect and sensitized the cells to UV-induced apoptosis, most notably at 10 and 20 J/m2 (Figure 5b). This effect is most likely the consequence of impaired DNA repair owing to reduced p15PAF expression, as the cells that do not efficiently repair their DNA are more sensitive to UV-induced cell death. Accordingly, caspase activation is clearly induced when p15PAF expression is inhibited (Figure 5c). To better comprehend how ATF3 and p15PAF may have opposing effects on cell survival while belonging to the same pathway, we knocked down ATF3 or Hif2a in UVC-irradiated (20 J/m2) cells deficient for p15PAF expression (Figure 5c). The results clearly showed that the inhibition of both ATF3 and Hif2a strongly reduced the caspase activation mediated by p15PAF inhibition. Hif2a induction by ATF3 is therefore required to mediate apoptosis as soon as the cells accumulate DNA damage. We further assessed the effect of ectopic expressions of ATF3 and p15PAF on cell survival, under both basal conditions and in cells irradiated with 20 J/m2 UVC. Although the ectopic expression of ATF3 displayed detrimental effect on cell survival in both conditions, the transfection of p15PAF did not compromise cell survival in basal conditions, and provided protection against UV-mediated cell death. Interestingly, the functional invalidation of Hif2a in the context of cells that overexpress ATF3 in UV-irradiated condition gave rise to a survival effect comparable with what was observed with p15PAF overexpression (Figure 5d). It is therefore possible to mimic the p15PAF effect with ATF3 expression by maintaining low Hif2a expression levels. Taken together, our results clearly indicate that the opposite effect of ATF3 on cell survival is due to its contribution to the regulation of Hif2a expression. By controlling p15PAF and Hif2a expressions, ATF3 is therefore able to contribute to the orchestration of DNA repair and cell death in maintenance of cellular integrity.
ATF3 and p15PAF protein expressions correlate with CPD production. CPDs, ATF3 and p15PAF were detected by immunofluorescence (shown in red). Nuclear labeling with DAPI is shown in blue. Correlation between CPD production, ATF3 and p15PAF expression is shown according to a time course after UVC irradiation. HaCaT cells were irradiated with a single low dose of 5 J/m2 UVC. A colour version of this Figure is available online
ATF3 and p15PAF are required for the DNA repair process. (a) HaCaT cells were transfected with siLuc, sip15PAF or siATF3. After 24 h, the cells were UVC irradiated (5 J/m2), and the presence of CPDs was determined by immunofluorescence after a recovery time of 24 h. The expressions of ATF3 and p15PAF in the presence or absence of their respective siRNA were monitored 2 h after irradiation by immunofluorescence (a) and western blot (b). (c) Quantification was performed by counting the CPD-positive cells. A total of 500 were counted two independent times. Statistical analysis was performed (Student t-test): the ***P-value represents a significant difference between siLuc and siATF3 or sip15PAF-transfected cells. (d) HaCaT cells were transfected with siLuc and siATF3 or sip15PAF. After 24 h of transfection, the cells were UVC-irradiated or not. At 2 and 24 h after irradiation, genomic DNA was extracted and subjected to a PCR stop assay as described in the Materials and Methods section. Amplification efficiency is inversely proportional to the extent of DNA damage. The experiment was performed three independent times in duplicate
ATF3 and p15PAF have opposing effects on cell survival. (a) HeLa cells were exposed to increasing doses of UVC (5–40 J/m2). Total RNA was extracted for 1 h and 30 min after irradiation and subjected to QPCR analysis to determine levels of p15PAF and Hif2a. (b) HeLa cells were transfected with siLuc, siATF3 or siP15PAF. At 24 h after transfection, the cells were irradiated with increasing doses of UVC (5–80 J/m2). After 24 h, the percentage of living cells was determined by trypan blue staining. (c) HeLa cells were transfected with siLuc, siP15PAF or a combination of siP15PAF/siATF3, or siP15PAF/siHif2a. After 24 h, the cells were either left untreated or irradiated with 20 J/m2 UVC. The activation of caspase 3/7 was determined using the caspase glo assay as described in the Materials and Methods section. (d) HeLa cells were transfected with either p15PAF- or ATF3-expressing constructs or a combination of the ATF3-expressing construct with siHif2a. At 24 h after transfection, the cells were either left untreated or irradiated with 20 J/m2 UVC. After 24 h, the percentage of living cells was determined by trypan blue staining. These experiments were performed three independent times in duplicate
p15PAF is a major effector of ATF3 in the mediation of DNA repair
It is possible to quantify the extent of DNA damage by using a reporter luciferase gene in which the intensity of the luciferase values is inversely proportional to the damage. A SV40LUC plasmid was exposed to high doses of UVC radiation (1000 J/m2) to induce CPDs. Damaged and undamaged SV40LUC constructs were then co-transfected into WT and ATF3 knockout (KO) MEF cells along with ATF3- or p15PAF-expressing vectors, or empty pcDNA3 or pEGFP plasmids as controls. The day after transfection, the cells were irradiated with a single low dose of UVC (5 J/m2) to trigger endogenous DNA repair mechanisms. After 24 h recovery, the luciferase activity was assessed. Irradiated WT MEF cells efficiently repaired the damaged SV40LUC plasmid (Figure 6a), whereas ATF3 KO MEF cells failed to do so (Figure 6b). However, exogenous ATF3 or p15PAF expression was able to significantly rescue the luciferase activity (Figure 6b). Interestingly, ectopic expression of either ATF3 or p15PAF expression in WT MEFs is sufficient to mediate repair of the damaged SV40LUC construct under basal conditions (Figure 6c). The expressions of ATF3 and p15PAF in KO MEF cells were confirmed by western blot (Figure 6d). Our results confirm the requirement of ATF3 and p15PAF expressions for efficient DNA repair process and suggest p15PAF as a major effector, as its expression is sufficient to compensate for the lack of ATF3. To test the possibility that the expressions of ATF3 and p15PAF could be sufficient to trigger DNA repair mechanisms in epithelial cells, the same experimental setting was performed in nonirradiated HeLa cells. Both ATF3 and p15PAF expressions significantly restored the damaged SV40LUC construct (Figure 7a). In contrast, empty pcDNA3 or GFP expression failed to induce cellular repair mechanisms. The expressions of ATF3 or p15PAF are therefore sufficient to trigger DNA repair. Conversely, knock down of ATF3 or p15PAF during UV irradiation by the transfection of appropriate siRNAs (siATF3, sip15PAF and a mutated version of siLuc unable to target the luciferase mRNA) is sufficient to impair the repair of the damaged reporter gene (Figure 7b). To confirm whether or not p15PAF constitutes a major effector of ATF3 for DNA repair in epithelial cells, we transfected both damaged and undamaged SV40LUC constructs in HeLa cells, along with ATF3- and p15PAF-expressing vectors in the presence of sip15PAF. Knock down of p15PAF drastically impaired ATF3-mediated DNA repair (Figure 7a), thus showing that p15PAF not only belongs to the ATF3 pathway but also constitutes one of its major effectors in the mediation of DNA repair. Accordingly, knock down of endogenous ATF3 or p15PAF failed to repair the damaged SV40LUC reporter construct (Figure 7b). The expression and efficiency of sip15PAF were monitored by QPCR (Figure 7c).
ATF3 and p15PAF are able to trigger DNA repair. (a) WT MEF cells were transfected with an SV40 luciferase reporter gene (white) or an SV40 luciferase construct damaged by 1000 J/m2 UVC exposure (black). At 24 h after transfection, the cells were irradiated with a single low dose of UVC (5 J/m2) to trigger endogenous DNA repair mechanism. ATF3- or p15PAF-expressing constructs, or pcDNA3 and pEGFP control constructs, were transfected into ATF3 KO MEF cells (b) or WT MEF cells (c) along with an SV40 luciferase reporter gene undamaged (white) or damaged (black) by 1000 J/m2 UVC irradiation. At 24 h after transfection, ATF3 KO MEF cells were irradiated with a single low dose of UVC (5 J/m2). The luciferase values are inversely proportional to the DNA damage. These experiments have been performed three independent times in quadruplicate. (d) The efficiency of ATF3 and p15PAF transfection was assessed by western blot
p15PAF is a major effector of ATF3 for mediating the DNA repair process. (a) Damaged SV40luc has been transfected in HeLa cells, along with p15PAF, ATF3-expressing constructs or pcDNA3 and pEGFP as control constructs in the presence of siLuc unable to trigger the luciferase (black) or sip15PAF (gray). The day after transfection, the luciferase activity was assessed. (b) HeLa cells have been co-transfected by siLuc, siATF3, sip15PAF along with damaged or undamaged SV40luc construct. After 24 h, the cells have been irradiated or not by 5 J/m2 UVC. In these experiments, siLuc has been mutated to prevent the degradation of luciferase mRNA. These experiments have been performed three independent times in quadruplicates. (c) ATF3 and p15PAF expressions as well as sip15PAF and siATF3 efficiencies have been controlled by QPCR
Interaction between p15PAFand PCNA is required for the DNA repair process
PCNA is part of a key protein complex involved in the repair of UV-induced DNA damage. This complex is necessary for filling the gap left by the NER machinery.15 The physical UV-induced association between p15PAF and PCNA occurs through a PCNA-binding domain in p15PAF, and has been described earlier.16, 17 To determine whether p15PAF-mediated DNA repair requires association with PCNA and thus acts through PCNA, we sought to inhibit this association and evaluate the DNA repair efficiency. Interestingly, it has been reported17 that p21cip1 competes with p15PAF for binding to PCNA, and the expression of >2-fold p21cip1 protein is sufficient to totally abolish PCNA and p15PAF association.17 To confirm such competition in our model, under basal conditions or in cells irradiated with 20 J/m2 UVC, knock down of p21 was performed in HeLa cells by the transfection of an siRNA specific to p21 (sip21). siLuc was transfected as a control. After lysis, proteins were immunoprecipitated using an anti-PCNA antibody and subsequently blotted with an antibody raised against p15PAF. Total protein extracts were subjected to western blotting using antibodies raised against p21 and β-tubulin as a loading control. The results showed a clear increase in p21 expression upon UV exposure, which was efficiently inhibited when the cells were transfected with sip21 (Figure 8a, right panel). The efficiency of sip21 is also clear in the nonirradiated condition (Figure 8a left panel). As expected and consistent with earlier published studies,17 p21 knockdown lead to a strong increase in p15PAF binding to PCNA under both basal and UV-irradiated conditions. Our results thus confirm the competition between p15PAF and p21 for binding to PCNA.
DNA repair depends on the interaction between p15PAF and PCNA. (a) HeLa cells were transfected with sip21 or siLuc as control. After 24 h, the cells were irradiated (right panel) or not (left panel) with 20 J/m2 UVC. At 3 h after irradiation, cells were lysed and proteins extracted. Protein extracts were then either subjected to western blotting with the appropriate anti-p21cip or anti-β-tubulin (loading control) antibodies (upper panel), or immunoprecipitated using anti-PCNA and subsequently blotted using anti-p15PAF antibodies (lower panel). (b) Undamaged SV40luc and UVC-damaged SV40luc reporter gene constructs were co-transfected into HeLa cells along with WT p21 or p21ΔPIP. After 24 h, the cells were UVC-irradiated to trigger endogenous DNA repair mechanisms. Luciferase expression was detected 24 h after irradiation. (c) Undamaged SV40luc and UVC-damaged SV40luc reporter gene constructs were transfected into HeLa cells along with p15PAF, ATF3-, WT p21- or p21ΔPIP-expressing constructs. After 24 h, luciferase activity was assessed. (d) HeLa cells were transfected with the UVC-damaged SV40luc construct along with p15PAF, or WT p21 and increasing amounts of p15PAF. Luciferase expression was assayed 24 h after transfection. These experiments have been performed three independent times in quadruplicate
Next, HeLa cells were transfected with a damaged SV40LUC reporter gene along with either a WT p21cip1 construct or, as a control, a mutated p21cip1 (p21ΔPIP) unable to associate with PCNA because of the deletion of the PIP domain. The day after transfection, DNA repair mechanisms were triggered by a single low dose of UVC (5 J/m2) (Figure 8b). Although efficient repair was observed in the cells transfected with the p21ΔPIP construct, overexpression of WT p21cip1 clearly impaired the DNA repair process. Similar results were observed for ATF3- and p15PAF-mediated DNA repair without UVC exposure (Figure 8c). Conversely, increasing levels of p15PAF are able to neutralize the negative impact of WT p21cip1 (Figure 8d). Neither WT p21cip1 p21ΔPIP nor ATF3 and p15PAF displayed any effects on the luciferase activity obtained with the transfection of undamaged SV40luc reporter gene. The expression of WT p21cip1 and the deleted construct p21ΔPIP in HeLa cells were confirmed by western blot (Supplementary Figure 2). Indeed, our results show that the association between p15PAF and PCNA is crucial for DNA repair and, therefore, p15PAF constitutes a key factor that allows proper PCNA function.
Discussion
Our results show for the first time the contribution of ATF3 in the global genomic repair of UV-damaged epithelial cells. The fact that the inhibition of ATF3 impairs both cell death9, 10, 11 and DNA repair strongly suggests this transcription factor as an essential early regulator of epithelial homeostasis during UV stress response. We further show that p15PAF constitutes a major effector of ATF3 for DNA repair. Although p15PAF downregulation seems to be dependent on the p53–p21 pathway,18 we show here, that after UV treatment, ATF3 is directly responsible for p15PAF expression. p15PAF also known as PCNA-associated factor, KIAA0101 or OEATC-1,16, 17 has been shown to localize to the mitochondria and the nucleus.16 Its expression is induced upon UV irradiation and it localizes in the cell in the same multiprotein complex as the tumor suppressor p33ING1B.16 p15PAF binds to PCNA and this binding is enhanced after UV irradiation.16, 17 It is possible that this association, which prevents the binding of p21cip1 to PCNA,17 may lead to p21 efficient ubiquitin-dependent degradation, an essential event for optimal DNA repair through PCNA function.19 Indeed, recent studies in various cellular models clearly show that downregulation or degradation of p21 after low doses of UV irradiation is required to allow proper DNA repair,20, 21, 22, 23 whereas a nondegradable mutant of p21 is able to efficiently block this process.19 Conversely, overexpression of p21cip1 is able to inhibit, by competing for the same PCNA-binding domain (PIP domain), the association of p15PAF with PCNA. Here we show that inhibition of such interaction impairs not only p15PAF but also ATF3-mediated DNA repair. Therefore, the mechanism of action of p15PAF is linked, at least in part, to PCNA function. After the removal of damage by the NER machinery, the DNA polymerases, Pol-δ and Pol-ɛ, are involved in the synthesis of the new DNA fragment. PCNA is required for this process, as it acts as a processivity factor for both polymerases.15 Interestingly, p15PAF has been found to interact with both PCNA and Pol δ18 and therefore may contribute, as cofactor bound to PCNA, to the polymerase recruitment. Combined with the fact that reduced p15PAF expression or inhibition of its binding to PCNA alters DNA repair, p15PAF may be considered an essential cofactor in the DNA damage response.
In melanoma cells, ATF3 has been shown to behave as a pro-cancerous factor by increasing metastatic potential.24 Furthermore, a potential dichotomous function has been reported, as ATF3 promotes survival of MCF10CA1a breast cancer cells while enhancing their motility.25 However, although these findings support a role for ATF3 as an oncogenic factor, recent cancer profiling array studies that compared 154 microdissected human tumors and their corresponding normal tissues,26 as well as the conclusions of the Cancer Genome Anatomy Project (http://cgap.nci.nih.gov), confirm that the overall expression level of ATF3 in cancers is statistically lower than in normal tissues. It is therefore tempting to speculate that the lack of ATF3 expression contributes to the impaired DNA damage response responsible for oncogenesis. Although upregulation and oncogenic function of p15PAF has been described in esophageal tumors17 and in pancreatic cancer,18 a putative tumor suppressor function has been suggested by its downregulation in several human colorectal tumors and hepatocellular carcinoma.16, 27 As the downregulation does not appear to be related to mutations,16 it is possible that reduction in the levels of p15PAF is mediated by the lack of ATF3 expression. The discrepancy in expression parallels the apparent opposite effects of ATF3 observed in human cancers, and suggests that both p15PAF and ATF3 expression and function may be differently regulated depending on the tumor type.
In conclusion, our results suggest ATF3 and p15PAF as novel gatekeepers of anticancer barrier mechanisms by contributing to the orchestration of DNA repair of damaged cells. Alteration of this pathway during the onset of the genotoxic stress response may therefore have deleterious consequences on genomic integrity.
Materials and Methods
Cell culture
Cells were cultivated at 37°C in 5% CO2. MEFs derived from ATF3-null mice9 were obtained from Tsonwin Hai (Center for Molecular Neurobiology, The Ohio State University, Columbus, Ohio, USA). Immortalized keratinocytes, HaCaT cells, HeLa and MEFs were maintained in the Dulbecco's modified Eagle's medium supplemented with 10% bovine fetal serum.
Immunofluorescence
Cells were fixed in 5% formaldehyde for 20 min at room temperature and incubated for 1 h at room temperature with an anti-ATF3 antibody (1:100) (C19-SC188, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or p15(PAF) antibody (1:100).16 For anti-CPD staining, cells were incubated for 5 min in 2 N HCl at room temperature and incubated with anti-CPD (1:2000; D194-1, MBL, Nagoya, Japan). For nuclei labeling, cells were incubated with a DAPI reagent (10 ng/ml) for 10 min at room temperature.
Transient transfection
ATF3- and p15PAF-expressing constructs have been described earlier.16, 28 Transfection experiments were carried out in 24-well plate (60 000) cells, using the Lipofectamine 2000 reagent (Invitrogen). When indicated, cells were UVC-irradiated (5 J/m2, Vilber Lourmat) the day after transfection. Transfection of the siRNAs was performed using the Lipofectamine 2000 reagent (0.1 μM siRNA with 20 μl lipofectamine). HaCaT or HeLa cells were plated 1 day before transfection in 60 mm wells at a density of 800 000 cells per dish. The sequences of siRNAs are as follows: siLuc, CGUACGCGGAAUACUUCGGAdTdT; siLuc-mut, CGUACGGCAGGCUGUUCGAdTdT; siATF3, GCACCUCUGCCACCGGAUGdTdT; siP15PAF, AAAGCAGACAGUGUUCCAGGCdTdT; and sip21, GAUGGAACUUCGACUUUGUdTdT.
Transcriptome, QPCR and western blot
The human pan genomic array from the Reseau National des Genopoles (RNG) was used as described.29 Real-time one-step RT-PCR and western blot were performed as described elsewhere.7 ATF3, p15PAF and ERK2 were detected using polyclonal antibodies to ATF3 (Sc-188; Santa Cruz Biotechnology Inc.), mouse monoclonal antibody to αERK2 D-2 (Sc-1647; Santa Cruz Biotechnology Inc.) or rabbit polyclonal antibody to p15PAF.16
Immunoprecipitation
HeLa cells transfected with siRNA p21 or siRNA control (siLuc) were either left untreated or irradiated with 20 J/m2 UVC. After 3 h, the cells were lysed in the extraction/immunoprecipitation buffer containing 25 mM Hepes, pH 7.2, 0.5 M NaCl, 1 mM EDTA, 0.5% Triton X-100, 10% glycerol, 5 mM MgCl2 and a cocktail of protease-phosphatase inhibitors. Immunoprecipitation was performed on 300 μg of protein extract. Samples were pre-cleared with protein G-coupled sepharose (Amersham) for 15 min at 4°C, then incubated for 2 h on ice with an anti-PCNA (PC-10, Santa Cruz Biotechnology Inc.) antibody (1 μg/ml). The samples were washed thrice with the extraction buffer and then precipitated for 1 h with protein G-coupled sepharose at 4°C. After washing, the samples were denatured for 15 min in the Laemmli buffer at 65°C before loading on 15% acrylamide gels. Proteins were electrotransferred to the PVDF Immobilon membrane (Millipore), then western blotted with anti-p1516 and anti-p21 (C-19, Santa Cruz) antibodies (1:1000 and 1:200, respectively).
ChIP assay
ChIP assay was performed as described.7 The size of the chromatin was an average of 1 kb. The identification of the captured p15PAF regulatory sequences was performed by PCR using appropriate primers P1 (L: 5′-TCGCCCAGGCTGGAGTGCAT-3′; 5′-CGTCTTCTTGAGAACCGAATGGTGTCA-3′), P2 (L: 5′-CCTGGGGCGCCTACGAATCC-3′; 5′-CCCCCGCCCTCCAGTACCAC-3′) and the nonrelevant primers P3 (L: 5′-GGCCCCTCCGGGAAACTGTG-3′; 5′-AATGCCAGCCCCAGCGTCAA-3′).
Analysis of DNA repair
UV adduct formation and repair were analyzed by a PCR-based DNA damage assay (PCR stop assay).14 HaCaT cells (106 cells in 60 mm dishes) were either left untreated or UVC-irradiated (5 J/m2). After 2 and 24 h, a 2-kb region of HPRT gene (primer available upon request) was amplified by PCR (20 cycles) from genomic DNA. As an internal control, a 170-bp region of the same gene was amplified. The smaller region represents a target too small to register significant levels of damage under these conditions. The intensity of the PCR product was determined with the ImageJ application. The intensity of the long-amplified HPRT fragment was normalized to the intensity of the small-amplified region.
For the gene reporter experiments, we exposed a SV40 luciferase reporter construct (PGL3 control) to massive doses of UVC irradiation (1000 J/m2). This damaged plasmid was then transfected along with ATF3, p15PAF, EGFP, empty vectors or appropriate siRNA. The same experiment setting was used with an undamaged SV40 plasmid as control. The day after transfection, the cells were lysed and the luciferase activity was measured.
Abbreviations
- ATF3:
-
activating transcription factor 3
- CPD:
-
cyclobutane pyrimidine dimmers
- EGR1:
-
early growth response 1
- Hif-2α:
-
hypoxia inducible factor 2α
- MEF:
-
mouse embryonic fibroblast
- NER:
-
nucleotide excision repair
- PAF:
-
proliferating cell nuclear antigen-associated factor
- TTD:
-
trichothiodystrophy
- UV:
-
ultraviolet
- XP:
-
xeroderma pigmentosum
References
Mocquet V, Laine JP, Riedl T, Yajin Z, Lee MY, Egly JM . Sequential recruitment of the repair factors during NER: the role of XPG in initiating the resynthesis step. EMBO J 2008; 27: 155–167.
Harper EG, Alvares SM, Carter WG . Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J Cell Sci 2005; 118: 3471–3485.
Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene 2002; 21: 7488–7496.
Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol 2004; 24: 5721–5732.
Kool J, Hamdi M, Cornelissen-Steijger P, van der Eb AJ, Terleth C, van Dam H . Induction of ATF3 by ionizing radiation is mediated via a signaling pathway that includes ATM, Nibrin1, stress-induced MAPkinases and ATF-2. Oncogene 2003; 22: 4235–4242.
Hai T, Hartman MG . The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 2001; 273: 1–11.
Thyss R, Virolle V, Imbert V, Peyron JF, Aberdam D, Virolle T . NF-kappaB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J 2005; 24: 128–137.
Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T et al. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol 2001; 3: 1124–1128.
Lu D, Wolfgang CD, Hai T . Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem 2006; 281: 10473–10481.
Turchi L, Aberdam E, Mazure N, Pouyssegur J, Deckert M, Kitajima S et al. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ 2008; 15: 1472–1480.
Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD . Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther 2005; 4: 233–241.
Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P . Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 2004; 279: 5288–5297.
Mizutani K, Onda M, Asaka S, Akaishi J, Miyamoto S, Yoshida A et al. Overexpressed in anaplastic thyroid carcinoma-1 (OEATC-1) as a novel gene responsible for anaplastic thyroid carcinoma. Cancer 2005; 103: 1785–1790.
Jennerwein MM, Eastman A . A polymerase chain reaction-based method to detect cisplatin adducts in specific genes. Nucleic Acids Res 1991; 19: 6209–6214.
Costa RM, Chigancas V, Galhardo Rda S, Carvalho H, Menck CF . The eukaryotic nucleotide excision repair pathway. Biochimie 2003; 85: 1083–1099.
Simpson F, Lammerts van Bueren K, Butterfield N, Bennetts JS, Bowles J, Adolphe C et al. The PCNA-associated factor KIAA0101/p15(PAF) binds the potential tumor suppressor product p33ING1b. Exp Cell Res 2006; 312: 73–85.
Yu P, Huang B, Shen M, Lau C, Chan E, Michel J et al. p15(PAF), a novel PCNA associated factor with increased expression in tumor tissues. Oncogene 2001; 20: 484–489.
Hosokawa M, Takehara A, Matsuda K, Eguchi H, Ohigashi H, Ishikawa O et al. Oncogenic role of KIAA0101 interacting with proliferating cell nuclear antigen in pancreatic cancer. Cancer Res 2007; 67: 2568–2576.
Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A et al. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell 2003; 114: 599–610.
Maeda T, Espino RA, Chomey EG, Luong L, Bano A, Meakins D et al. Loss of p21WAF1/Cip1 in Gadd45-deficient keratinocytes restores DNA repair capacity. Carcinogenesis 2005; 26: 1804–1810.
Soria G, Podhajcer O, Prives C, Gottifredi V . P21Cip1/WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation. Oncogene 2006; 25: 2829–2838.
Soria G, Speroni J, Podhajcer OL, Prives C, Gottifredi V . p21 differentially regulates DNA replication and DNA-repair-associated processes after UV irradiation. J Cell Sci 2008; 121: 3271–3282.
Stoyanova T, Yoon T, Kopanja D, Mokyr MB, Raychaudhuri P . The xeroderma pigmentosum group E gene product DDB2 activates nucleotide excision repair by regulating the level of p21Waf1/Cip1. Mol Cell Biol 2008; 28: 177–187.
Ishiguro T, Naito M, Hanaoka K, Nagawa H, Muto T, Tsuruo T . Enhanced metastasis of B16 melanoma cells by unexpected elevated expression of the metastasis-associated TI-241 (LRF-1-, Jun-Fos-related) gene treated with antisense oligonucleotide. Clin Exp Metastasis 1998; 16: 179–183.
Yin X, Dewille JW, Hai T . A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene 2008; 27: 2118–2127.
Yan C, Boyd DD . ATF3 regulates the stability of p53: a link to cancer. Cell Cycle 2006; 5: 926–929.
Guo M, Li J, Wan D, Gu J . KIAA0101 (OEACT-1), an expressionally down-regulated and growth-inhibitory gene in human hepatocellular carcinoma. BMC Cancer 2006; 6: 109.
Kawauchi J, Zhang C, Nobori K, Hashimoto Y, Adachi MT, Noda A et al. Transcriptional repressor activating transcription factor 3 protects human umbilical vein endothelial cells from tumor necrosis factor-alpha-induced apoptosis through down-regulation of p53 transcription. J Biol Chem 2002; 277: 39025–39034.
Le Brigand K, Russell R, Moreilhon C, Rouillard JM, Jost B, Amiot F et al. An open-access long oligonucleotide microarray resource for analysis of the human and mouse transcriptomes. Nucleic Acids Res 2006; 34: e87.
Acknowledgements
The authors thank Professor Tsonwin Hai for the gift of the immortalized ATF3−/− mouse embryonic fibroblast and matching wild-type cells. We thank Pr Ducommun for the gift of the p21 and p21ΔPIP constructs. The authors also thank Dr. Pascal Barbry and Virginie Virolle-de Thillot (IPMC, Sophia Antipolis) for the transcriptome analysis. This work was supported by grants from the Association pour la Recherche sur le Cancer, the Société de Recherche en Dermatologie, the Institut National de la Santé et de la Recherche Medicale, the Institut National du Cancer, and the Australian National Health and Medical Research Council (to CW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M Blagosklonny
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Turchi, L., Fareh, M., Aberdam, E. et al. ATF3 and p15PAF are novel gatekeepers of genomic integrity upon UV stress. Cell Death Differ 16, 728–737 (2009). https://doi.org/10.1038/cdd.2009.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2009.2
Keywords
This article is cited by
-
PCNA-associated factor (KIAA0101/PCLAF) overexpression and gene copy number alterations in hepatocellular carcinoma tissues
BMC Cancer (2021)
-
Synergistic effect of PAF inhibition and X-ray irradiation in non-small cell lung cancer cells
Strahlentherapie und Onkologie (2021)
-
Atf3 deficiency promotes genome instability and spontaneous tumorigenesis in mice
Oncogene (2018)
-
The stress-responsive gene ATF3 regulates the histone acetyltransferase Tip60
Nature Communications (2015)
-
NS5ATP9 Suppresses Activation of Human Hepatic Stellate Cells, Possibly via Inhibition of Smad3/Phosphorylated-Smad3 Expression
Inflammation (2015)