Abstract
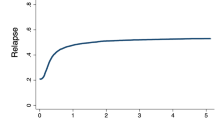
Although the role of autologous hematopoietic cell transplantation (auto-HCT) is well established in neuroblastoma (NBL), the role of allogeneic HCT (allo-HCT) is controversial. The Center for International Blood and Marrow Transplant Research conducted a retrospective review of 143 allo-HCT for NBL reported in 1990–2007. Patients were categorized into two different groups: those who had not (Group 1) and had (Group 2) undergone a prior auto-HCT (n=46 and 97, respectively). One-year and five-year OS were 59% and 29% for Group 1 and 50% and 7% for Group 2, respectively. Among donor types, disease-free survival (DFS) and OS were significantly lower for unrelated transplants at 1 and 3 years but not at 5 years post HCT. Patients in CR or very good partial response (VGPR) at transplant had lower relapse rates and better DFS and OS, compared with those not in CR or VGPR. Our analysis indicates that allo-HCT can cure some neuroblastoma patients, with lower relapse rates and improved survival in patients without a history of prior auto-HCT as compared with those patients who had previously undergone auto-HCT. Although the data do not address why either strategy was chosen for patients, allo-HCT after a prior auto-HCT appears to offer minimal benefit. Disease recurrence remains the most common cause of treatment failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fish JD, Grupp SA . Stem cell transplantation for neuroblastoma. Bone Marrow Transplant 2008; 41: 159–165.
Maris JM, Hogarty MD, Bagatell R, Cohn SL . Neuroblastoma. Lancet 2007; 369: 2106–2120.
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 1999; 341: 1165–1173.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol 2009; 27: 1007–1013.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363: 1324–1334.
Matthay KK, Seeger RC, Reynolds CP, Stram DO, O'Leary MC, Harris RE et al. Allogeneic versus autologous purged bone marrow transplantation for neuroblastoma: a report from the Childrens Cancer Group. J Clin Oncol 1994; 12: 2382–2389.
Philip T, Ladenstein R, Lasset C, Hartmann O, Zucker JM, Pinkerton R et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur J Cancer 1997; 33: 2130–2135.
Ladenstein R, Potschger U, Hartman O, Pearson AD, Klingebiel T, Castel V et al. 28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures. Bone Marrow Transplant 2008; 41 (Suppl 2): S118–S127.
Jubert C, Wall DA, Grimley M, Champagne MA, Duval M . Engraftment of unrelated cord blood after reduced-intensity conditioning regimen in children with refractory neuroblastoma: a feasibility trial. Bone Marrow Transplant 2011; 46: 232–237.
Strullu M, Rialland F, Cahu X, Brissot E, Corradini N, Thomas C et al. Allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning regimen in children: a single-center experience. Eur J Haematol 2012; 88: 504–509.
Pession A, Masetti R, Di Leo C, Franzoni M, Prete A . HLA-mismatched hematopoietic stem cell tranplantation for pediatric solid tumors. Pediatr Rep 2011; 3 (Suppl 2): e12.
Sung KW, Park JE, Chueh HW, Lee SH, Yoo KH, Koo HH et al. Reduced-intensity allogeneic stem cell transplantation for children with neuroblastoma who failed tandem autologous stem cell transplantation. Pediatr Blood Cancer 2011; 57: 660–665.
Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008; 14: 748–758.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15: 367–369.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Atkinson K, Horowitz MM, Gale RP, van Bekkum DW, Gluckman E, Good RA et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood 1990; 75: 2459–2464.
George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol 2006; 24: 2891–2896.
Grupp SA, Stern JW, Bunin N, Nancarrow C, Ross AA, Mogul M et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol 2000; 18: 2567–2575.
Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol 2002; 20: 2284–2292.
Rill DR, Santana VM, Roberts WM, Nilson T, Bowman LC, Krance RA et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood 1994; 84: 380–383.
Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study. J Clin Oncol 2011; 29: 4351–4357.
Kreissman SG, Villablanca JG, Diller L, London WB, Maris JM, Park JR et al. Response and toxicity to a dose-intensive multi-agent chemotherapy induction regimen for high risk neuroblastoma (HR-NB): a Children's Oncology Group (COG A 3973) study. J Clin Oncol 2007; 25: 9505.
Takahashi Y, Matsumoto K, Fujisaki H, Iwasaki F, Hashii Y, Nakamura K et al. Unrelated cord blood transplantation for children with high-risk or relapsed neuroblastoma. Bone Marrow Transpl 2012; 47: S47.
Inoue M, Nakano T, Yoneda A, Nishikawa M, Nakayama M, Yumura-Yagi K et al. Graft-versus-tumor effect in a patient with advanced neuroblastoma who received HLA haplo-identical bone marrow transplantation. Bone Marrow Transplant 2003; 32: 103–106.
Ash S, Stein J, Askenasy N, Yaniv I . Immunomodulation with dendritic cells and donor lymphocyte infusion converge to induce graft vs neuroblastoma reactions without GVHD after allogeneic bone marrow transplantation. Br J Cancer 2010; 103: 1597–1605.
Ash S, Gigi V, Askenasy N, Fabian I, Stein J, Yaniv I . Graft versus neuroblastoma reaction is efficiently elicited by allogeneic bone marrow transplantation through cytolytic activity in the absence of GVHD. Cancer Immunol Immunother 2009; 58: 2073–2084.
Toporski J, Garkavij M, Tennvall J, Ora I, Gleisner KS, Dykes JH et al. High-dose iodine-131-metaiodobenzylguanidine with haploidentical stem cell transplantation and posttransplant immunotherapy in children with relapsed/refractory neuroblastoma. Biol Blood Marrow Transplant 2009; 15: 1077–1085.
Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res 2010; 70: 9554–9561.
Venstrom JM, Zheng J, Noor N, Danis KE, Yeh AW, Cheung IY et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res 2009; 15: 7330–7334.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia and Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hale, G., Arora, M., Ahn, K. et al. Allogeneic hematopoietic cell transplantation for neuroblastoma: the CIBMTR experience. Bone Marrow Transplant 48, 1056–1064 (2013). https://doi.org/10.1038/bmt.2012.284
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.284
Keywords
This article is cited by
-
Differentiation of early relapse and late relapse in intermediate- and high-risk neuroblastoma with an 18F-FDG PET/CT-based radiomics nomogram
Abdominal Radiology (2024)
-
Targeting GD2 after allogeneic SCT: effector cell composition defines the optimal use of ch14.18 and the bispecific antibody construct NG-CU (GD2-CD3)
Cancer Immunology, Immunotherapy (2023)
-
Stammzelltransplantation bei pädiatrischen soliden Tumoren
Der Onkologe (2021)
-
Reduction of myeloid-derived suppressor cells reinforces the anti-solid tumor effect of recipient leukocyte infusion in murine neuroblastoma-bearing allogeneic bone marrow chimeras
Cancer Immunology, Immunotherapy (2018)