Abstract
Background:
Docetaxel is the only FDA-approved first-line treatment for castration-resistant prostate cancer (CRPC) patients. Docetaxel treatment inevitably leads to tumour recurrence after an initial therapeutic response with generation of multinucleated polyploid (MP) cells. Here we investigated role of MP cells in clinical relapse of CRPC.
Methods:
Prostate cancer (PC-3) cells were treated with docetaxel (5 nM) for 3 days followed by a washout and samples were collected at close intervals over 35 days post drug washout. The tumorigenic potential of the giant MP cells was studied by implanting MP cells subcutaneously as tumour xenografts in nude mice.
Results:
Docetaxel-induced polyploid cells undergo mitotic slippage and eventually spawn mononucleated cells via asymmetric cell division or neosis. Both MP and cells derived from polyploid cells had increased survival signals, were positive for CD44 and were resistant to docetaxel chemotherapy. Although MP cells were tumorigenic in nude mice, these cells took a significantly longer time to form tumours compared with parent PC-3 cells.
Conclusions:
Generation of MP cells upon docetaxel therapy is an adaptive response of apoptosis-reluctant cells. These giant cells ultimately contribute to the generation of mononucleated aneuploid cells via neosis and may have a fundamental role precipitating clinical relapse and chemoresistance in CRPC.
Similar content being viewed by others
Main
Despite significant advances in research, diagnosis, and clinical practice, prostate cancer still remains the second most commonly diagnosed cancer and the sixth most common cause of cancer-related death among men worldwide (Attard et al, 2011). Androgen-deprivation remains the mainstay of the first-line treatment for both primary and metastatic prostate cancer. Initially, majority of the patients respond well to this treatment but eventually the tumour progresses to castration-resistant prostate cancer (CRPC), which is the major cause of mortality (Tong et al, 2016). Docetaxel was approved by the European Medicine Agency and the US Food and Drug Administration in 2004 for the first-line treatment of patients with CRPC and is now the only standard of care in this setting (Hussain et al, 1999; Tannock et al, 2004). Although this clinical regimen prolongs overall survival in CRPC patients, the cancer unfortunately recurs (clinical relapse) inevitably after an initial illusionary therapeutic response. Currently, there is a lack of mechanistic knowledge underlying this tumour cell ‘replenishment’ after docetaxel treatment, which inevitably leads to tumour recurrence and translates to only a modest increase in overall survival. The present study explores this therapy-relapse paradox that inescapably results in tumour recurrence shortly after a therapy response.
The formation of giant multinucleated polyploid (MP) cells after therapeutic intervention with either taxane-based chemotherapy including docetaxel or DNA-damaging agents has been well described. Studies have reported that some tumour microenvironmental factors including hypoxia are also responsible for the generation of MP cells. Studies have also shown that hypoxia-mimicking CoCl2 treatment induces formation of polyploid cells that contributes to expansion of a cell subpopulation with stem cell characteristics (Lopez-Sanchez et al, 2014; Zhang et al, 2014a). These polyploid cells can be a result of DNA over-replication (Nakayama et al, 2009), abrogated mitotic checkpoint (Erenpreisa et al, 2008), or failed cytokinesis (Sagona and Stenmark, 2010). It was long assumed that these giant polyploid cells do not survive and die due to ‘mitotic catastrophe’ subsequent to multipolar cell division. However, recent evidence indicated that although most polyploid cells succumb to cell death, a small percentage of them survive and produce viable progeny (Erenpreisa and Cragg, 2007; Coward and Harding, 2014). A study also found that colon cancer cells treated with DNA-damaging agent cisplatin generated giant polyploid cells, a subset of which were able to engender viable clones via asymmetric cell division; furthermore, this phenomenon was recapitulated in an in vivo xenograft model of colon cancer treated with cisplatin (Puig et al, 2008). Another study revealed that when PC-3 cells were treated with docetaxel, it led to growth arrest and formation of multinucleated cells and the generation of docetaxel-resistant progeny (Makarovskiy et al, 2002). A very recent study has reported the cabazitaxel, a second-line chemotherapy in CRPC treatment, also leads to chemoresistance by inducing severe multinucleation in prostate cancer cells (Martin et al, 2016). Altogether, these data suggest that polyploid cells that were once presumed to be either destined for terminal growth arrest or cell death may actually represent a ‘transition state’ for generation of viable clones.
The current study aimed to analyse the long-term effects of post docetaxel exposure on prostate cancer cells. Our study shows that most of the prostate cancer cells exposed to docetaxel undergo cell death following mitotic arrest. However, a small percentage of the cells ‘slip out’ of mitosis to form giant MP cells. Most of these MP cells succumbed to cell death, but a small fraction survived for several weeks, eventually giving rise to small mononucleated cells via an asymmetric cell division process called neosis. We further show that these MP cells have tumorigenic potential in nude mice, and that both MP cells and cells derived from MP cells (CDPC) are chemoresistant and were positive for cancer stem cell (CSC) marker CD44. In conclusion, the formation of MP cells after docetaxel treatment suggests an escape process that is involved in tumour relapse and chemoresistance following an initial illusionary therapeutic response.
Materials and methods
Cell culture and treatment schedule
PC-3 and DU145 cells were obtained from American Type Cell Culture (Manassas, VA, USA) and were grown in RPMI medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were maintained in humidified 5% CO2. All experiments were performed using 5 nM of docetaxel unless stated otherwise.
Flow cytometry
Cells were harvested at different time intervals, washed twice with ice-cold PBS, and fixed in 70% ethanol for at least 24 h. Cell pellets were then washed with PBS followed by RNase A (2 mg ml−1) addition and staining with anti-MPM-2 primary antibody and Alexa-488-conjugated secondary antibody. Propidium iodide (0.1% in 0.6% Triton X-100 in PBS) was added for 45 min in dark followed by analysis on a FACS Canto flow-cytometer (BD Canto, San Jose, CA, USA) using FlowJo software (Ashland, OR, USA).
Immunofluorescence
Cells were grown on glass coverslips for immunofluorescence microscopy and were fixed and blocked as described previously (Mittal et al, 2015). Coverslips were incubated in primary antibodies (1 : 2000 dilution) against γ-tubulin and α-tubulin at 1 : 2000 dilution for 1 h at 37 °C, washed with 1 × PBS for 10 min at room temperature, and then incubated in 1 : 2000 Alexa 488- or 555-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA). Cells were washed 5 × with PBS and then mounted with Prolong-Gold antifade reagent that contained DAPI (Invitrogen).
Microscopy
Immunofluorescently stained cells were imaged utilizing the Zeiss LSC 700 confocal microscope (Oberkochen, Germany) and were processed with Zen software (Oberkochen).
Time-lapse imaging
Giant MP cells were isolated and plated 12 days after docetaxel removal. Cells were imaged for 7 days using time-lapse microscopy at × 40 magnification on Zeiss Axio Observer 5A (Oberkochen). Differential Interface Contrast Images were captured at multiple points every 2 h for 7 days and were processed with Zen software (Oberkochen). Magnifications and details related to imaging are provided in individual sections.
Lysate preparation and immunoblotting
Cells were cultured to ∼80% confluence and after treatments as mentioned in individual sections protein lysates were prepared as described previously (Mittal et al, 2016). Polyacrylamide gel electrophoresis was used to resolve the proteins, which were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The immune reactive bands were visualized by using Pierce ECL chemiluminescence detection kit (Thermo Fischer, Waltham, MA, USA). Primary Antibodies, Phospho-Bcl2 (Thr 56) human, Bcl-XL (54H6), Survivin (71G4B7), Beclin-2, and CD44 (156-3C11) were obtained from Cell Signaling (Danvers, MA, USA). β-actin (SC47778 from Santa Cruz Biotechnology, Dallas, TX, USA) was used as loading control. All relative band intensities were quantitated by densitometry and were normalized against β-actin values using ImageJ (Schneider et al, 2012).
Cell migration assay using Boyden chambers
A total of 10 000 cells suspended in RPMI medium containing 0.5% FBS were added to the upper well of the Boyden chamber. The lower chamber was filled with RPMI medium containing 10% FBS. After 48 h, cells that had migrated to the bottom surface of the filter were fixed with 70% methanol, stained with crystal violet, and counted under a microscope in 10 randomly selected fields at a magnification of × 20.
MTT assay
MTT assay was used to measure metabolic activity suitable for analysis of proliferation rates between PC-3, giant MP, and CDPC. Approximately 10 000 cells of each cell type were seeded into each well of a 96-well microplate. The assay was performed over a 6-day period with incubation times at 24, 48, 72, 96, 120, and 144 h. At the end of each incubation period, 10 μl of MTT (Sigma-Aldrich, St Louis, MO, USA) at 5 mg ml−1 in PBS was added into each well after removal of the culture medium and incubated for 4 h under the same conditions. After the incubation, 100 μl of DMSO was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a 96-well microplate reader.
In vivo tumour growth
A total of 50 000 PC-3 cells or giant MP cells (cells that were treated with 5 nM docetaxel and harvested 1 day after drug removal) were subcutaneously injected in the right flank of 6-week old male BALB/c nude mice (Harlan Laboratories, Indianapolis, IN, USA). Tumours were measured every week using a digital Vernier Caliper. The two longest perpendicular axes in the x/y plane of each xenograft tumour were measured to the nearest 0.1 mm. The depth was assumed to be equivalent to the shortest of the perpendicular axes, defined as y. Tumour volume was calculated using the formulae xy2/2 as is standard practice. All animal experiments were performed in compliance with Georgia State University (GSU) Institutional Animal Care and Use Committee (IACUC) guidelines. All animal protocols (including description of experiments and experimenters) were approved by GSU IACUC.
Statistical analyses
Unless otherwise stated in the Methods and Results sections, statistical analyses were performed using two-tailed Student’s t-tests. The criterion for statistical significance for all analyses was P<0.05.
Results
Docetaxel induces formation of giant MPcells
To corroborate the fact that docetaxel treatment causes mitotic arrest before cell death, we examined the cell cycle events post docetaxel treatment. To this end, we treated PC-3 cells with 5 nM docetaxel for 72 h and then stained them for MPM-2 antibody (mitotic cell marker) by flow cytometry. As shown in Figure 1A, there was an induction of mitotic arrest 24 h post docetaxel treatment. Using immunofluorescence confocal microscopy, we noticed large number of mitotically arrested cells (Figure 1B) displaying aberrant multipolar spindles 24 h after docetaxel treatment. On the other hand, DMSO-treated control cells showed normal bipolar cell division (Figure 1B and Supplementary Figure 4). Following this, a disappearance of the M-phase population and an emergence of apoptotic cells (sub-G1 population) was observed at 48 h after docetaxel treatment (Figure 1D).
Docetaxel induces formation of giant MP cells.(A) Cell-cycle histograms of doubly stained PC-3 cells treated with docetaxel at 5 nM concentration for different time points showing mitotic arrest and slippage at different time points. (B) Representative immunofluorescent confocal micrographs of PC-3 cells treated with docetaxel for 24 and 72 h, indicating mitotic arrest and emergence of giant MP cells respectively. Centrosomes and microtubules were immunolabeled for γ-tubulin (green) and α-tubulin (red), respectively, and DNA was counterstained with DAPI (blue). Scale bar (white), 5 μm. (C) Cell-cycle histogram of docetaxel treated PC-3 cells showing emergence of polyploid population. (D) Bar-graphs showing the percentage of sub-G1 and mitotic population resulting from 5 nM docetaxel treatment. (E) Bar-graphs showing the percentage of giant MP cells 72 h after docetaxel treatment.
We next investigated whether docetaxel in addition to causing mitotic arrest and cell death induces other phenotypic changes. At 72 h post treatment, there was an emergence of G1/G2-interphase cells, which in addition to being much bigger in size were also multinucleated compared with parent PC-3 cells (Figure 1B). In addition, we observed that there was a significant drop in the percentage of MPM-2 positive cells from ∼11% at 48 h to 1% at 72 h and a simultaneous increase in MPM-2 negative population from ∼26% at 24 h to 42% at 72 h (Figure 1A and D). Most probably, these large multinucleated cells resulted from a mitotic exit, that is, cells slipping out of mitosis without cell division. As cells have failed to successfully progress through mitosis to execute cytokinesis, they have twice or more the amount of DNA as compared to parent PC-3 cell in the G1 phase of the cell cycle (Figure 1C). We termed these pseudo-G1-like cells as giant MP cells. Almost 95% of the surviving cells after 3 days of docetaxel treatment were giant MP cells. Taken together, these observations clearly suggest that, upon docetaxel treatment a small percentage of cells slip out of mitosis resulting in the formation of giant MP cells. The induction of giant MP cells was not limited to PC-3 cells but was also formed in two other cell lines: DU-145 (androgen-dependent prostate cancer cell line) and MDA-MB-231 (triple-negative breast cancer cell line) (Supplementary Figure 1).
Giant MP cells undergo asymmetric cell division via neosis
Having established that docetaxel treatment induces the formation of giant MP cells, we next followed the fate of these giant MP cells for the consequent 35 days after docetaxel removal. To accomplish this, we collected cells every third day post drug removal and, employing confocal microscopy, we visualized the morphology of PC-3 cells. Microtubules were immunolabelled for α-tubulin (red) and DNA was counterstained with DAPI (blue). For the first 15 days post drug removal, we did not observe any remarkable changes in cell morphology. After 15 days in culture, we started observing the formation of small mononucleated cells in the vicinity of giant MP cells (Figure 2A). The number of mononucleated cells further increased by day 25 (Figure 2A and E). At later time points (day 35) following the removal of docetaxel, sparse colonies of small, tightly-packed, mononucleated cells were observed in the culture dish (Figure 2A and E).
Giant MP cells undergo asymmetric cell division via neosis.(A) Confocal micrographs of docetaxel treated PC-3 cells. Cells are stained with α-tubulin (red) and DNA (blue) and showed emergence of small mononucleated cells on different days (D) after drug removal. Scale bar (white) 5 μm. (B) Time lapse images of giant MP cells generating small-sized daughter cells via budding (black arrows) over a 7-day period. Scale bar (black), 5 μm. (C) Confocal immunographs of cells stained with α-tubulin (red) and DNA (blue) showing transport of DNA from the branches of the giant MP cells (white arrows). (D) Line graph representing the total number of small sized nucleated cells and giant MP cells at different days after drug removal.
We next examined the origin of these mononucleated cells at day 15 after removal of docetaxel. To this end, starting at 12 days after docetaxel removal, we isolated and plated three giant MP cells per well by serial dilution and followed them for 7 days using time lapse imaging. We observed asymmetric cell division pattern in giant MP cells through a process known as neosis (Navolanic et al, 2004; Sundaram et al, 2004; Rajaraman et al, 2005). Small mononucleated daughter cells were seen budding from the giant MP cells from the branches of the giant MP cells (Figure 2B). To confirm the presence of DNA in the budding cells, we stained the giant MP cell along with the budding cells with Hoechst 33342 and used it in combination with differential interference contrast microscopy. Using this method, we were able to detect the presence of DNA in the budding daughter cells (Supplementary Figure 2). We named these budding cells as CDPCs. Furthermore, by using confocal microscopy, we demonstrated that the DNA was transported within the branches of the giant MP cells (Figure 2C). These results demonstrate that giant MP cells produce daughter cells through a process of budding also known as neosis where the branches of the giant MP cells can serve as vessels for DNA transport.
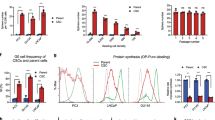
Giant MP cells and CDPCs are chemoresistant
After establishing that giant MP cells can survive and can form small mononucleated cells via neosis, we next determined their response to docetaxel treatment. For this purpose, PC-3, CDPC, and giant MP cells were treated with 5 nM of docetaxel for 48 h. Cell death was estimated by measuring the expression of cleaved caspase-3 and cleaved PARP. Interestingly, we observed increased levels of cleaved caspase-3 staining in PC-3 cells after 48 h of docetaxel treatment (Figure 3A). This was further substantiated by increased protein expression of cleaved PARP and cleaved caspase-3 by immunoblotting (Figure 3B and Supplementary Table 1). On the other hand, CDPC and giant MP cells showed lower expression of both cleaved caspase-3 and cleaved PARP after docetaxel exposure, suggesting that these cells were resistant to docetaxel treatment.
Giant MP cells and CDPC are chemoresistant.(A) Confocal images of cleaved caspase-3 staining (red) on docetaxel treated PC-3 cells. (B) Representative immunoblot images of cleaved caspase-3 and cleaved-PARP for docetaxel treated cells. Actin was used as the loading control. (C) Graphical representation of cell survival using MTT assay. Cells were treated with 3 different concentrations of docetaxel and MTT assay was done 48 h after docetaxel treatment. (D) Western blottings of anti-apoptotic and survival proteins in at different days after docetaxel removal. D0 are control PC-3 cells while cells on D35 are CDPCs.
Next, we evaluated the sensitivity of PC-3, CDPC, and giant MP cells to various concentrations of docetaxel (5, 10, and 20 nM) using MTT assay. PC-3 cells exhibited a dose-dependent increase in cell death 48 h after docetaxel treatment when compared with, both CDPC and giant MP cells (Figure 3C). In addition, we assessed the expression of anti-apoptotic and survival proteins in both CDPC and giant MP cells and compared them with the parental cells. The expression of anti-apoptotic proteins like Bcl-2, pBcl-2 and Bcl-XL were much higher in giant MP cells (day 1 to day 25 in Figure 3D and Supplementary Table 2) and CDPC (day 35) as compared with parental PC-3 cells (Day0). Similarly, survival proteins such as survivin and beclin-2 were also higher in giant MP cells and CDPC compared with parent PC-3 cells. Taken together, these results suggested that both giant MP cells and CDPC exhibited a completely different response to docetaxel treatment as compared to the parental cells owing to the increased expression of anti-apoptotic and survival proteins. We also observed that the giant MP and CDPC cells were positive for CD44, which is a CSC marker (Supplementary Figure 3E and Supplementary Table 4).
Giant MP cells and CDPC show differential ability to migrate and proliferate as compared to parent PC-3 cells
We next examined the ability of the giant MP cells and CDPC to migrate and proliferate and compared it to the parent PC-3 cells. Transwell migration assay was used to compare the migratory ability of CDPC and giant MP cells with that of the parent PC-3 cells. At 48 h, the mean number of parent PC-3 cells that had migrated across the membrane was ninefold higher than the giant MP cells and twofold higher than the CDPC cells (Figure 4A and B). The MTT assay showed the giant MP cells showed no signs of proliferation over a 6-day period. On the other hand, the proliferation rate of CDPC was similar to that of the parent PC-3 cells over the 6-day period (Figure 4C). Surprisingly, the mesenchymal marker vimentin was much higher in CDPC and giant MP as compared with the parent PC-3 cells (Figure 4D and Supplementary Table 3). We speculate that the giant MP cells use this mesenchymal phenotype to transport DNA through the branches in order to produce small mononucleated cells via neosis (Figure 2D). These results suggest that giant MP cells have slow proliferation and low rates of invasion and tumour formation compared to the parent PC-3 cells, suggesting that giant MP cells are less aggressive or malignant than that of parental cells.
Phenotypic changes in CDPC and giant MP cells.(A) Bright-field microscopic images of cells stained with crystal violet showing invasion capacity of PC-3, CDPC, and giant MP cells. (B) Bar graph representing the number of migrated cells in a Boyden chamber. (C) Cell proliferation assay over a 7-day period using MTT assay. (D) Representative immunoblots of Vimentin in PC-3, giant MP, and CDPC’s. (E and F) Graphical representation of the percent aneuploidy and number chromosomes respectively using fluorescent in situ hybridization analysis. A total of 50 cells were counted.
We next wanted to see whether the CDPCs have a different genetic profile as compared with the parent PC-3 cells. We measured the degree of aneuploidy in the CDPC and parent PC-3 cells using fluorescent in situ hybridization (Figure 4E). The average number chromosomes in the parent PC-3 cells were 83 as compared with 82 in the CDPC (Figure 4F). We next calculated the percent aneuploidy in parent PC-3 and CDPCs by counting the number of cells that had either<80 or >86 chromosomes. Using this method, the percent aneuploidy in parent PC-3 cells was 12% compared with 18% in CDPC. Taken together the degree of aneuploidy in CDPC was comparable to that of the parent PC-3 cells (e.g., chromosomal translocation). As there was a high number of translocations/duplications/and so on in the parent PC-3 cells, making many of the chromosomes unidentifiable, we were not able to measure the degree of structural chromosomal abnormalities in the PC-3 and CDPC cells. These results suggested that the degree of aneuploidy in the parent PC-3 and CDPC was not very different.
Giant MP cells have tumorigenic potential
To test the tumorigenicity of giant MP cells, we collected the giant MP cells 3 days after docetaxel treatment. A total of 50 000 PC-3 or giant MP cells were injected into the right flank of the nude mice (n=6). As shown in Figure 5A, 6/6 mice (100%) injected with PC-3 cells formed tumours, whereas only 2/6 mice (33%) injected with giant MP cells formed tumours in the nude mice. We also measured the kinetics of the tumour growth over a period of 60 days. Measurable tumours started to form as early as 15 days post inoculation in mice injected with parent PC-3 cells. On the other hand, measurable tumours were started to form only after 28 days’ post inoculation in mice injected with giant MP cells. The rate of tumour growth was also much higher in mice injected with PC-3 cells compared with mice that were injected with giant MP cells (Figure 5C). At the end of 60 days, the tumour weight of mice injected with PC-3 cells was three times higher than the tumours formed by giant MP cells (Figure 5B and C). These results suggested that even though the giant MP cells have tumorigenic capacity, the rate of tumour formation is much slower than the parent PC-3 cells.
Giant MP cells have tumorigenic potential.(A) Bar graph representing the number of animals forming tumours after injecting either PC-3 or giant MP cells. (B) Bar graph representing the tumour weight. (C) Tumour growth monitored (by Vernier calipers) and presented as tumour volume in cubic millimeter over a period of 60 days.
Discussion
Docetaxel, a member of the taxane class of antimicrotubule agents is the only FDA-approved chemotherapeutic agent for CRPC. In many clinical situations, tumours will initially respond successfully to docetaxel but subsequently relapse and become progressively more malignant. This is mainly because of the intermittent dosing schedule followed for docetaxel administration. This intermittent dosing regimen allows tumour regrowth between treatment schedules resulting in only a partial regression of the prostate tumour mass. Our current study very elegantly demonstrates that the formation of giant MP cells due to docetaxel treatment is the culprit cell population responsible for cancer relapse. We describe here that the formation of giant MP cells is mainly a result of mitotic slippage. It is well known that the mitotically slipped cells can undergo a second round of DNA replication without undergoing mitosis leading to formation of giant MP cells (Galan-Malo et al, 2012). This phenomenon is known as endoduplication (Edgar and Orr-Weaver, 2001; Lee et al, 2009). Several reports suggest that p53 is an important component of ploidy checkpoint and its overexpression to can lead endoduplication of cell (Aylon and Oren, 2011). In addition, generation of resistant giant multinucleated cells has been reported in p53-mutated tumour cells (Illidge et al, 2000). As PC-3 cells lack p53 (Rubin et al, 1991), endoduplication and subsequent formation of giant MP cells may be attributed to the loss of p53 gene.
These giant MP cells can not only survive for a long period of time but could give rise to small mononucleated, actively proliferating cells that can later cause the tumour to relapse. Here, the giant MP cells undergo a novel type of cell division that involves nuclear budding followed by intracellular cytokinesis, to produce mononucleated daughter cells that ‘bud off’ from the giant MP cell, a phenomenon known as neosis or reductive cell division. Previous studies have also shown that giant MP cells can form small daughter cells through this process of neosis (Sundaram et al, 2004; Rajaraman et al, 2005, 2006; Puig et al, 2008; Coward and Harding, 2014; Zhang et al, 2014b). These mononucleated daughter cells have previously been reported to be highly error-prone, which increases the rates of genomic instability and contributes to tumour regrowth (Chen et al, 2016).
We further show that giant MP cells and cells generated from them (CDPC) are chemoresistant. The fact the giant MP cells are chemoresistant was not surprising. It is well known that tumour cells in patients’ bodies are present in different phases of the cell cycle (G1, S, G2, and M) and conventional chemotherapy kills only the most vulnerable phase of the cell cycle but spares the others (Abal et al, 2003). In other words, the cell death upon administration of the chemotherapeutic agent depends upon the ‘cytotoxicity window’ of the drug (Abal et al, 2003). In particular, extensive literature reports that the high ‘cytotoxicity window’ of docetaxel corresponds to late S and G2 phase of the cell cycle (Hennequin et al, 1995; Paoletti et al, 1997). Studies have convincingly demonstrated that cells are variably sensitive to docetaxel when treatment is applied to cells synchronized in different phases of the cell cycle. It is now well established that docetaxel is almost totally lethal against S-phase cells, but is only partially toxic against cells in G1 phase of the cell cycle. As these giant MP cells are pseudo-G1-like cells, these cells are very unlikely to die upon docetaxel treatment. We also speculate that the chemoresistance of CDPC cells could be attributed in part to high expression of antiapoptotic (cIAP-1, cIAP-2, XIAP, survivin, and clusterin) (McEleny et al, 2004) proteins and survival proteins (Bcl-2 and Bcl-XL) (Feldman and Feldman, 2001; O'Neill et al, 2011). It is also likely to be that CDPCs have altered expression of drug transporter proteins, thus making them less susceptible to docetaxel toxicity.(Aberuyi et al, 2014; Rahgozar et al, 2014; de Morree et al, 2016; Ma et al, 2016)
These cells also express CSC marker CD44. This CSC marker has a critical role in regulating the properties of CSCs such as, tumour initiation, self-renewal, and chemoradioresistance (Yan et al, 2015). Our findings are in line with a previous study, which reported that docetaxel-resistant prostate cancer cells exhibit increased stemness compared with their parental counterpart (Puhr et al, 2012). Thus, targeting CSCs seems to be potential mechanism to combat the resistance and relapse developed after docetaxel treatment. In line with this, a recent study showed that Napabucasin (BBI608), a cancer cell stemness inhibitor, suppresses the prostate cancer growth and makes the prostate cancer cells sensitive to docetaxel by killing the prostate CSCs that were resistant to docetaxel (Zhang et al, 2016). Thus, we speculate that the docetaxel treatment leads to clonal selection of highly aggressive phenotype with stem cell-like phenotype and this in part is responsible for the chemotherapy failure.
Finally, we show evidence that giant MP cells have tumorigenic potential in nude mice. The giant MP cells take a significantly longer time to form the tumour as compared with the parent PC-3 cells. This is because the giant MP cells first need to produce the mononucleated daughter cells which then subsequently proliferate to form the tumour. This result is in concordance to the fact that tumour relapse always occurs after a significant delay usually ranging from a couple of months to sometimes years.
Taken together, our studies show that generation of giant MP cells that were once presumed to be either destined for terminal growth arrest or cell death may actually represent a ‘transition state’ for generation of viable clones. These cells may have an integral part in tumour relapse and generation of chemoresistance. Strategies preventing the formation of giant MP cells and understanding the molecular mechanism of neosis will help us identify key targets to prevent tumour relapse.
Change history
25 April 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abal M, Andreu JM, Barasoain I (2003) Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 3 (3): 193–203.
Aberuyi N, Rahgozar S, Moafi A (2014) The role of ATP-binding cassette transporter A2 in childhood acute lymphoblastic leukemia multidrug resistance. Iran J Ped Hematol Oncol 4 (3): 118–126.
Attard G, Richards J, de Bono JS (2011) New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res 17 (7): 1649–1657.
Aylon Y, Oren M (2011) p53: guardian of ploidy. Mol Oncol 5 (4): 315–323.
Chen S, Stout JR, Dharmaiah S, Yde S, Calvi BR, Walczak CE (2016) Transient endoreplication down-regulates the kinesin-14 HSET and contributes to genomic instability. Mol Biol Cell 27 (19): 2911–2923.
Coward J, Harding A (2014) Size does matter: why polyploid tumor cells are critical drug targets in the war on cancer. Front Oncol 4: 123.
de Morree ES, Bottcher R, van Soest RJ, Aghai A, de Ridder CM, Gibson AA, Mathijssen RH, Burger H, Wiemer EA, Sparreboom A, de Wit R, van Weerden WM (2016) Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br J Cancer 115 (6): 674–681.
Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105 (3): 297–306.
Erenpreisa J, Cragg MS (2007) Cancer: a matter of life cycle? Cell Biol Int 31 (12): 1507–1510.
Erenpreisa J, Ivanov A, Wheatley SP, Kosmacek EA, Ianzini F, Anisimov AP, Mackey M, Davis PJ, Plakhins G, Illidge TM (2008) Endopolyploidy in irradiated p53-deficient tumour cell lines: persistence of cell division activity in giant cells expressing Aurora-B kinase. Cell Biol Int 32 (9): 1044–1056.
Feldman BJ, Feldman D (2001) The development of androgen-independent prostate cancer. Nat Rev Cancer 1 (1): 34–45.
Galan-Malo P, Vela L, Gonzalo O, Calvo-Sanjuan R, Gracia-Fleta L, Naval J, Marzo I (2012) Cell fate after mitotic arrest in different tumor cells is determined by the balance between slippage and apoptotic threshold. Toxicol Appl Pharmacol 258 (3): 384–393.
Hennequin C, Giocanti N, Favaudon V (1995) S-phase specificity of cell killing by docetaxel (Taxotere) in synchronised HeLa cells. Br J Cancer 71 (6): 1194–1198.
Hussain M, Petrylak D, Fisher E, Tangen C, Crawford D (1999) Docetaxel (Taxotere) and estramustine versus mitoxantrone and prednisone for hormone-refractory prostate cancer: scientific basis and design of Southwest Oncology Group Study 9916. Semin Oncol 26 (5 Suppl 17): 55–60.
Illidge TM, Cragg MS, Fringes B, Olive P, Erenpreisa JA (2000) Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell Biol Int 24 (9): 621–633.
Lee HO, Davidson JM, Duronio RJ (2009) Endoreplication: polyploidy with purpose. Genes Dev 23 (21): 2461–2477.
Lopez-Sanchez LM, Jimenez C, Valverde A, Hernandez V, Penarando J, Martinez A, Lopez-Pedrera C, Munoz-Castaneda JR, De la Haba-Rodriguez JR, Aranda E, Rodriguez-Ariza A (2014) CoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancer. PLoS One 9 (6): e99143.
Ma Y, Miao Y, Peng Z, Sandgren J, De Stahl TD, Huss M, Lennartsson L, Liu Y, Nister M, Nilsson S, Li C (2016) Identification of mutations, gene expression changes and fusion transcripts by whole transcriptome RNAseq in docetaxel resistant prostate cancer cells. SpringerPlus 5 (1): 1861.
Makarovskiy AN, Siryaporn E, Hixson DC, Akerley W (2002) Survival of docetaxel-resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cell Mol Life Sci 59 (7): 1198–1211.
Martin SK, Pu H, Penticuff JC, Cao Z, Horbinski C, Kyprianou N (2016) Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res 76 (4): 912–926.
McEleny K, Coffey R, Morrissey C, Williamson K, Zangemeister-Wittke U, Fitzpatrick JM, Watson RW (2004) An antisense oligonucleotide to cIAP-1 sensitizes prostate cancer cells to fas and TNFalpha mediated apoptosis. Prostate 59 (4): 419–425.
Mittal K, Choi da H, Klimov S, Pawar S, Kaur R, Mitra AK, Gupta MV, Sams R, Cantuaria G, Rida PC, Aneja R (2016) A centrosome clustering protein, KIFC1, predicts aggressive disease course in serous ovarian adenocarcinomas. J Ovarian Res 9: 17.
Mittal K, Ogden A, Reid MD, Rida PC, Varambally S, Aneja R (2015) Amplified centrosomes may underlie aggressive disease course in pancreatic ductal adenocarcinoma. Cell Cycle 14 (17): 2798–2809.
Nakayama Y, Igarashi A, Kikuchi I, Obata Y, Fukumoto Y, Yamaguchi N (2009) Bleomycin-induced over-replication involves sustained inhibition of mitotic entry through the ATM/ATR pathway. Exp Cell Res 315 (15): 2515–2528.
Navolanic PM, Akula SM, McCubrey JA (2004) Neosis and its potential role in cancer development and chemoresistance. Cancer Biol Ther 3 (2): 219–220.
O'Neill AJ, Prencipe M, Dowling C, Fan Y, Mulrane L, Gallagher WM, O'Connor D, O'Connor R, Devery A, Corcoran C, Rani S, O'Driscoll L, Fitzpatrick JM, Watson RW (2011) Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer 10: 126.
Paoletti A, Giocanti N, Favaudon V, Bornens M (1997) Pulse treatment of interphasic HeLa cells with nanomolar doses of docetaxel affects centrosome organization and leads to catastrophic exit of mitosis. J Cell Sci 110 (Pt 19): 2403–2415.
Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z (2012) Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol 181 (6): 2188–2201.
Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, Favier L, Ghiringhelli F, Kroemer G, Solary E, Martin F, Chauffert B (2008) Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol Int 32 (9): 1031–1043.
Rahgozar S, Moafi A, Abedi M, Entezar EGM, Moshtaghian J, Ghaedi K, Esmaeili A, Montazeri F (2014) mRNA expression profile of multidrug-resistant genes in acute lymphoblastic leukemia of children, a prognostic value for ABCA3 and ABCA2. Cancer Biol Ther 15 (1): 35–41.
Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR (2006) Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell Int 6: 25.
Rajaraman R, Rajaraman MM, Rajaraman SR, Guernsey DL (2005) Neosis—a paradigm of self-renewal in cancer. Cell Biol Int 29 (12): 1084–1097.
Rubin SJ, Hallahan DE, Ashman CR, Brachman DG, Beckett MA, Virudachalam S, Yandell DW, Weichselbaum RR (1991) Two prostate carcinoma cell lines demonstrate abnormalities in tumor suppressor genes. J Surg Oncol 46 (1): 31–36.
Sagona AP, Stenmark H (2010) Cytokinesis and cancer. FEBS Lett 584 (12): 2652–2661.
Schneider CA, Schneider WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9 (7): 671–675.
Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R (2004) Neosis: a novel type of cell division in cancer. Cancer Biol Ther 3 (2): 207–218.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal M, Eisenberger M (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351 (15): 1502–1512.
Tong D, Liu Q, Liu G, Yuan W, Wang L, Guo Y, Lan W, Zhang D, Dong S, Wang Y, Xiao H, Mu J, Mao C, Wong J, Jiang J (2016) The HIF/PHF8/AR axis promotes prostate cancer progression. Oncogenesis 5 (12): e283.
Yan Y, Zuo X, Wei D (2015) Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med 4 (9): 1033–1043.
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J (2014a) Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 33 (1): 116–128.
Zhang Y, Jin Z, Zhou H, Ou X, Xu Y, Li H, Liu C, Li B (2016) Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med 5 (6): 1251–1258.
Zhang YB, Wang X, Meister EA, Gong KR, Yan SC, Lu GW, Ji XM, Shao G (2014b) The effects of CoCl2 on HIF-1alpha protein under experimental conditions of autoprogressive hypoxia using mouse models. Int J Mol Sci 15 (6): 10999–11012.
Acknowledgements
We thank Dr Susan Olson and Nicole Owen from University of Oregon Health and Sciences University for helping us with the fluorescent in situ hybridization experiments. We gratefully acknowledge Noopur Bhatnagar, Leila Jahromiand and Brian D Melton for assisting with some experiments. This study was supported by grants to RA from the National Cancer Institutes of Health (U01 CA179671 and R01 CA169127) and a graduate fellowship to KM from the Second Century Initiative Program at Georgia State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Mittal, K., Donthamsetty, S., Kaur, R. et al. Multinucleated polyploidy drives resistance to Docetaxel chemotherapy in prostate cancer. Br J Cancer 116, 1186–1194 (2017). https://doi.org/10.1038/bjc.2017.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.78
Keywords
This article is cited by
-
Digital image analysis and machine learning-assisted prediction of neoadjuvant chemotherapy response in triple-negative breast cancer
Breast Cancer Research (2024)
-
Polyploidy and mTOR signaling: a possible molecular link
Cell Communication and Signaling (2024)
-
Serine/threonine kinase 36 induced epithelial-mesenchymal transition promotes docetaxel resistance in prostate cancer
Scientific Reports (2024)
-
Single-cell morphological and transcriptome analysis unveil inhibitors of polyploid giant breast cancer cells in vitro
Communications Biology (2023)
-
A mathematical investigation of polyaneuploid cancer cell memory and cross-resistance in state-structured cancer populations
Scientific Reports (2023)