Abstract
Background:
An increasing number and proportion of cancer patients with apparently localised disease are treated with chemotherapy and radiation therapy in contemporary oncology practice. In a pilot study of radiation-induced sarcoma (RIS) patients, we demonstrated that chemotherapy was associated with a reduced time to development of RIS. We now present a multi-centre collaborative study to validate this association.
Methods:
This was a retrospective cohort study of RIS cases across five large international sarcoma centres between 1 January 2000 to 31 December 2014. The primary endpoint was time to development of RIS.
Results:
We identified 419 patients with RIS. Chemotherapy for the first malignancy was associated with a shorter time to RIS development (HR 1.37; 95% CI: 1.08–1.72; P=0.009). In the multi-variable model, older age (HR 2.11; 95% CI 1.83–2.43; P<0.001) and chemotherapy for the first malignancy (HR 1.61; 95% CI 1.26–2.05; P<0·001) were independently associated with a shorter time to RIS. Anthracyclines and alkylating agents significantly contribute to the effect.
Conclusions:
This study confirms an association between chemotherapy given for the first malignancy and a shorter time to development of RIS.
Similar content being viewed by others
Main
A late complication of ionising radiation is the development of a secondary malignancy, most commonly a radiation-induced sarcoma (RIS). Reports of RIS date back to the early twentieth century, when bone sarcomas were described in Swiss watch dial painters who were exposed to radioactive phosphorus (Martland, 1929). RIS are typically high-grade and associated with a poor prognosis (Gladdy et al, 2010). They usually develop 10–20 years after radiation exposure (Mark et al, 1994), but have been reported as early as six months after radiation therapy (RT) (Cha et al, 2004).
The influence of chemotherapy on the pathogenesis of RIS is controversial. Several large population-based studies have shown an association between development of RIS and administration of chemotherapy for primary childhood cancers (Hawkins et al, 1996; Henderson et al, 2012). However, support for the role of chemotherapy treatment in adult cancers leading to RIS is less clear (Schaapveld et al, 2015). We previously reviewed the records of all sarcoma patients treated at the Bone and Soft Tissue Sarcoma Centre, Royal Prince Alfred Hospital in Sydney, Australia between June 2005 and January 2011 to examine the effects of chemotherapy on the latency of RIS. We reported a mean interval to RIS for patients who received chemotherapy with RT for their index cancer of 5 years, compared to 14.5 years in those receiving RT alone (P<0.005) (Suttie et al, 2012). We hypothesised that chemotherapy may enhance the effects of bone and soft tissue damage due to radiation, or interfere with DNA repair, thereby reducing the time to development of RIS, and conceivably increasing the incidence of RIS. The study reported here-in was undertaken to investigate whether concurrent chemotherapy was associated with the time to development of RIS in a larger and independent cohort.
Patients and methods
This was a retrospective cohort study of adult patients with RIS diagnosed from January 2000 to December 2014 at five large, tertiary referral sarcoma centres in the Asia-Pacific (National Cancer Center, Singapore), Europe (Royal Marsden Hospital, London; Royal Orthopaedic Hospital, Birmingham; Centre Léon Bérard, Lyon), and North America (Mount Sinai Hospital, Toronto). No patients were included from our original, hypothesis-generating Australian cohort (Suttie et al, 2012). We identified 419 patients with RIS, defined by (1) a history of radiation exposure at least 6 months before sarcoma diagnosis, (2) development of a sarcoma within the radiation field, and (3) pathological confirmation of a sarcoma that was histologically different from the first malignancy for which RT was given (Cahan et al, 1998).
The following demographic, clinical, and pathological information was collected from the databases: gender, age at diagnosis of the first malignancy, age at diagnosis of the RIS, site and histology of the first malignancy, treatment for the first malignancy (including RT and chemotherapy, delivered either concurrently or sequentially, with type and dose of treatment where possible), date of RIS diagnosis, site and histological subtype of RIS. Information on familial history and genetic results were also sought, but variably available.
The primary endpoint was the interval to development of RIS, defined as the interval from diagnosis of the first malignancy to diagnosis of the RIS. The relationships between chemotherapy, patient and cancer characteristics, and interval to RIS were assessed using Cox proportional hazards regression models to determine cause-specific hazard ratios (HRs). Reported P-values are two-sided, and P-values <0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics, version 23.
Based on earlier reports, we expected that one quarter of patients with RIS would have received chemotherapy for their first malignancy (exposed group) and that the mean difference in the time from RT to development of RIS in those exposed to chemotherapy vs not exposed to chemotherapy would be approximately 4–9 years (Newton et al, 1991; Suttie et al, 2012). In the Suttie et al study (12), the interval to RIS within each group was normally distributed with a standard deviation of 11.
If the true mean difference in the interval to RIS between those exposed to chemotherapy and those not exposed to chemotherapy was a mean of 5 years, then a sample size of approximately 204 (51 exposed vs 153 not exposed) would provide 80% power to reject the null hypothesis with a type 1 error of 0.05.
The study was approved by the Royal Prince Alfred Hospital Ethics Review Committee (Reference: X14-0415 & LNR/14/RPAH/552).
Results
Clinicopathological findings
In all, 419 patients with RIS were identified across all five centres. The clinicopathological characteristics of these patients are summarised in Table 1. The specific primary cancer diagnoses and RIS histologies are listed in Supplementary Table 1. The median age (range) at diagnosis of the first malignancy was 47 years (1–88), and of RIS was 63 years (16–92). There were more females (73%) than males (27%) with RIS. The most common first malignancies were breast (51%), head and neck (10%), and gynaecological cancers (9%). The most common RIS histotypes were angiosarcoma (28%) and osteosarcoma (17%). We did not see translocation-associated sarcomas, such as synovial sarcoma in this cohort.
All 419 patients were treated with RT for their first malignancy, as per the inclusion criteria, and 95 (23%) also received chemotherapy, either concurrently or sequentially. There were limited details of RT and chemotherapy for the index cancers. Radiation doses were available for 162 (39%) participants, in whom the median dose of radiotherapy for the first cancer was 50 Gy (range, 15–80). There was minimal information with regards to the modality and fractionation of radiation given. The most comprehensive information in regards to radiation doses was provided by the centre Léon Bérard, Mount Sinai Hospital and the National Cancer Centre. These groups contributed 160 cases of RIS to the database and radiation dose details were available for 111 of these cases.
Of the 95 patients who were known to receive chemotherapy for their first malignancy, information on the type of chemotherapy was available for 71 (75%) participants. Chemotherapy included three or more drugs in 57 (80%) patients, two drugs in 9 (13%), and one drug in 5 (7%).
Effect of chemotherapy on interval to RIS development
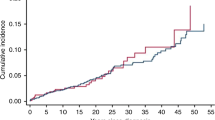
The median interval from the index cancer to development of RIS for the whole cohort was 11 years (range, 1–64, Table 1). Chemotherapy for the first malignancy was associated with a shorter interval to RIS (HR 1.37; 95% CI: 1.08–1.72; P=0.009; Figure 1A). The median interval to RIS in the chemotherapy group was 10 years (mean 12.3, range 3–39) vs 11.0 years (mean, 15.4; range, 0–57 years) for those not treated with chemotherapy for their first cancer. Age, gender and first cancer diagnosis were also associated with shorter interval to RIS development (Table 2). In the multi-variable analysis, older age (HR 2.11; 95% CI 1.83–2.43; P<0.001) and chemotherapy for the first malignancy (HR 1.61; 95% CI 1.26–2.05; P<0.001) were independently associated with a shorter interval to RIS (Table 2).
(A) Kaplan–Meier estimates of radiation-induced sarcoma-free survival by chemotherapy use for all patients in the study cohort; (B) Kaplan–Meier estimates of radiation-induced sarcoma-free survival by (B1) anthracycline and (B2) alkylating chemotherapy use in the Léon Bérard and Mount Sinai Hospital cohorts.
Role of chemotherapeutic agents on latency to RIS development
Léon Bérard and Mount Sinai Hospital provided the most comprehensive data with regards to primary chemotherapy and RT. Isolating their data of 125 patients, chemotherapy was again associated with a shortened time to development of RIS (HR 1.82; 95% CI: 1.24–2.69; P=0.002). Subjects treated with chemotherapy had a median interval to RIS development of 8 years compared with 14 years in those who were chemotherapy-free. The effect of anthracyclines in this group was striking. Of the 125 patients, 41 received chemotherapy for the primary malignancy and 84 did not receive chemotherapy. Of the 41 patients given chemotherapy, 28 (68%) had an anthracycline, 23 (56%) had an alkylating agent, and 38 (93%) had an anthracycline and/or alkylating agent (Supplementary Table 2). Patients given anthracyclines had a shorter interval to RIS than those not treated with an anthracycline (HR 2.56; 95% CI: 1.62–4.07; P<0.001; Figure 1B1). Similarly, treatment with an alkylating agent was also associated with a shorter interval to RIS (HR 1.57; 95% CI: 1.10–2.53; P=0·02; Figure 1B2). The dose of radiotherapy was not associated with interval to RIS.
Discussion
This study provides independent confirmation of the association between treatment with chemotherapy for an index cancer and a shorter interval to development of RIS. This finding is supportive of our previous study (Suttie et al, 2012). They are pertinent to people currently undergoing cancer treatment, of whom approximately 60% will be treated with RT (Delaney et al, 2003).
RIS is an established complication of RT, which appears to have a rising incidence (Thijssens et al, 2005). Other known risk factors for RIS include the dose of RT, younger age at initial diagnosis/treatment, and genetic susceptibility (Moppett et al, 2001). Modern cancer treatment algorithms frequently combine RT and chemotherapy. It is estimated that approximately 9% of cancer patients have an indication for chemotherapy combined with RT (Barton et al, 2013) and the use of this combined modality treatment, either sequentially or concurrently, is also increasing. This is particularly true for breast, rectal, and uterine cancers (Siegel et al, 2012; Miller et al, 2016), as well as for oesophageal and gastric cancers over the last decade (Trip et al, 2015).
In several studies of childhood cancers, treatment with chemotherapy plus RT has been shown to be associated with a higher incidence of subsequent RIS than treatment with RT alone. The evidence from these studies implicates alkylating agents and anthracyclines as the main contributors. However, the evidence is largely confined to the use of these agents combined with RT in childhood cancers. In the Late Effects Childhood Study, after adjusting for treatment with RT, the relative risk (RR) of developing a bone sarcoma after chemotherapy with an alkylating agent was 4.7 (95% CI: 1.0–22.3) (Tucker et al, 1987). Similarly, among 59 cases of radiation-induced bone cancers and 220 controls, there was a linear trend (P=0.08) in the RR of bone cancers with increasing doses of an alkylating agent (Hawkins et al, 1996). In the Childhood Cancer Survivor study, anthracycline use was associated with an RR of 3.5 for developing a secondary sarcoma (Henderson et al, 2012). In the Vu et al study of the risk of osteosarcoma after solid tumours during childhood, only exposure to alkylating agents, but not spindle or topoisomerase II inhibitors (presumably including anthracyclines), was associated with an increased risk of a secondary sarcoma (Le Vu et al, 1998).
In adult cancers, the associations between chemotherapy on RIS are less clear. A meta-analysis of breast RIS did not demonstrate an association with chemotherapy (Sheth et al, 2012). However, in a recent study of 3905 people aged 15–50 years treated with RT for Hodgkin’s disease, the risk of developing a second solid malignant neoplasm was increased in those patients treated with procarbazine-containing chemotherapy (Schaapveld et al, 2015).
There is only one study outside our group that has examined the effect of chemotherapy on the interval to development of a second malignancy neoplasm (SMN). This was in a paediatric population of 91 children with bone sarcomas developing after a primary cancer and showed that treatment with both an anthracycline and RT was associated with a reduced interval to an SMN. In the absence of anthracyclines, alkylating agents were not associated with interval to SMN. Treatment with both agents shortened the interval to SMN by a mean of 4.4 years (Newton et al, 1991), a time-frame similar to our current findings. In contrast to this, we found that even treatment with an alkylating agent alone can also shorten the interval to RIS, albeit with a smaller magnitude of effect compared with anthracyclines. We did not see an association of other topoisomerase II inhibitors with interval to RIS.
In pre-clinical studies, anthracyclines and alkylating agents have been shown to induce neoplastic transformation by disruption of DNA function both in vitro and in vivo (Marquardt et al, 1976; Westendorf et al, 1983). Alkylating agents induce permanent DNA damage by the alkylation of DNA. Topoisomerase II inhibitors produce DNA strand breaks, which occur during replication. Cells die by apoptosis where there is irreparable DNA damage. However, if drug-induced DNA damage is insufficient to kill the cells, it can result in chromosomal alterations. Anthracyclines may be more likely to lead to RIS compared to other topoisomerase II inhibitors due to free radical formation. This is likely an important mechanism of action since attempts to develop less toxic analogues in order to reduce cardiotoxicity due to free radicals have all resulted in loss of anti-tumour efficacy (Chabner and Longo, 2011). Even in the absence of radiation, anthracyclines and alkylating agents have been associated with increased risks of developing other SMNs, such as breast (Henderson et al, 2016), lung (Swerdlow et al, 2001; Travis et al, 2002), and various gastrointestinal cancers (Nottage et al, 2012; Dores et al, 2014). Henderson et al reported that the associations between breast cancer and anthracycline and alkylating agent exposure, are probably dose-dependent. Unfortunately, we had insufficient detailed data on chemotherapy to assess the possible effects of dose, and dose-density on interval to RIS.
Our present study also demonstrates that gender, type of first malignancy, and age at diagnosis of the first malignancy were all associated with a shorter interval to RIS. However, on multivariable analysis, only the associations with age at diagnosis of the first malignancy and treatment with chemotherapy were independently significant. Fifty per cent of first malignancies in our study were breast cancers, and this group had the shortest interval to RIS. Breast cancers and sarcomas are both associated with Li Fraumeni syndrome, and related syndromes with mutations in the tumour suppressor gene TP53 (Li and Fraumeni, 1969; Schneider et al, 1993), which could be one explanation for this finding. Of note, older patients had a shorter interval to RIS, which may be attributable to age-related underlying impairment of DNA repair and immune dysregulation. Similarly, Biau et al showed that older patients with soft tissue sarcomas have worse outcomes beyond their differences in treatment (Biau et al, 2012). The relationship between older age at diagnosis of the first malignancy and shorter interval to RIS is partly artefactual, because older age is associated with a shorter possible duration of follow-up. The correlations between age, gender, type of first cancer, and exposure to chemotherapy were inherent in our observational study design and cannot be disentangled.
One of the potential confounding effects of this study is that patients may have been selected to receive combined modality treatment at diagnosis because they had a higher grade or more aggressive primary malignancy in the first place, compared to those who did not have chemotherapy. These patients may have had a germline mutation in certain tumour suppressor or DNA repair genes, which not only predisposed them to their aggressive primary cancer, but also placed them at higher risk and at a shorter time to development of an RIS. As part of our data collection, we requested information on primary tumour staging, family history and any known genetic mutations. However, given the antiquity of many of the records, this information for all patients could not be retrieved. We accept that in context of this retrospective cohort study, there may be other confounders that may influence time to RIS that was not measured or inadequately captured in this study.
Despite the limitations of this study, it is the largest published study on RIS, and the largest study providing evidence of an association between treatment with chemotherapy and shortened interval to RIS. It is the only study to demonstrate this effect in a predominantly adult population. The increasing use of chemotherapy and RT might both reduce the interval to RIS, and increase the incidence of radiation-induced malignancies. The most definitive evidence for a hypothesised effect of chemotherapy on the incidence of RIS and the interval to RIS would come from randomised trials comparing RT vs RT plus chemotherapy and we now plan a meta-analysis of such trials.
Although secondary sarcomas remain relatively rare, accounting for only 3% of all sarcomas diagnosed (Bjerkehagen et al, 2008), our data underscore the importance of vigilant surveillance for second malignancies after RT. This is particularly relevant for younger patients being treated with RT for their first malignancy, as they have a longer potential time to develop a second cancer, and also those with genetic predispositions, who already have an elevated risk of developing a second cancer. Further studies are needed to examine the interactions between genetic factors, RT, chemotherapy, and the pathogenesis of RIS.
Conclusion
This study supports our hypothesis of an association between chemotherapy and a shortened interval to development of RIS. Awareness of the risk, and the time to development of RIS, should be taken into account in cancer treatment decisions, particularly where the need for chemotherapy in addition to radiation therapy, is of debatable benefit.
Change history
25 July 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Barton M, Jacob S, Shafiq J, Wong K, Thompson S, Delaney G (2013) Review of optimal radiotherapy utilisation rates. CCORE, Ingham Institute for Applied Medical Research (IIAMR): Liverpool, NSW, Australia. Available at: http://inghaminstitute.org.au/sites/default/files/RTU%20Review%20Final%20Dec%202012%20v2%2019032013.pdf.
Biau DJ, Ferguson PC, Chung P, Griffin AM, Catton CN, O'Sullivan B, Wunder JS (2012) Local recurrence of localized soft tissue sarcoma: a new look at old predictors. Cancer 118 (23): 5867–5877.
Bjerkehagen B, Smeland S, Walberg L, Skjeldal S, Hall KS, Nesland JM, Smastuen MC, Fossa SD, Saeter G (2008) Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol 47 (8): 1475–1482.
Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL (1998) Sarcoma arising in irradiated bone: report of eleven cases. 1948. Cancer 82 (1): 8–34.
Cha C, Antonescu CR, Quan ML, Maru S, Brennan MF (2004) Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg 239 (6): 903–909, ; discussion 909-10.
Chabner B, Longo DL (2011) Cancer Chemotherapy and Biotherapy Principles and Practice. Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA.
Delaney GP, Jacob S, Featherstone C, Barton MB (2003) Radiotherapy in cancer care: estimating optimal utilisation from a review of evidence-based clinical guidelines. Collaboration for Cancer Outcomes Research and Evaluation (CCORE) Liverpool Hospital, Sydney, Australia.
Dores GM, Curtis RE, van Leeuwen FE, Stovall M, Hall P, Lynch CF, Smith SA, Weathers RE, Storm HH, Hodgson DC, Kleinerman RA, Joensuu H, Johannesen TB, Andersson M, Holowaty EJ, Kaijser M, Pukkala E, Vaalavirta L, Fossa SD, Langmark F, Travis LB, Fraumeni JF Jr, Aleman BM, Morton LM, Gilbert ES (2014) Pancreatic cancer risk after treatment of Hodgkin lymphoma. Ann Oncol 25 (10): 2073–2079.
Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, Brennan MF, Singer S (2010) Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol 28 (12): 2064–2069.
Hawkins MM, Wilson LM, Burton HS, Potok MH, Winter DL, Marsden HB, Stovall MA (1996) Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst 88 (5): 270–278.
Henderson TO, Moskowitz CS, Chou JF, Bradbury AR, Neglia JP, Dang CT, Onel K, Novetsky Friedman D, Bhatia S, Strong LC, Stovall M, Kenney LB, Barnea D, Lorenzi E, Hammond S, Leisenring WM, Robison LL, Armstrong GT, Diller LR, Oeffinger KC (2016) Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: a report from the Childhood Cancer Survivor Study. J Clin Oncol 34 (9): 910–918.
Henderson TO, Rajaraman P, Stovall M, Constine LS, Olive A, Smith SA, Mertens A, Meadows A, Neglia JP, Hammond S, Whitton J, Inskip PD, Robison LL, Diller L (2012) Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 84 (1): 224–230.
Le Vu B, de Vathaire F, Shamsaldin A, Hawkins MM, Grimaud E, Hardiman C, Diallo I, Vassal G, Bessa E, Campbell S, Panis X, Daly-Schveitzer N, Lagrange JL, Zucker JM, Eschwege F, Chavaudra J, Lemerle J (1998) Radiation dose, chemotherapy and risk of osteosarcoma after solid tumours during childhood. Int J Cancer 77 (3): 370–377.
Li FP, Fraumeni Jr JF (1969) Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med 71 (4): 747–752.
Mark RJ, Poen J, Tran LM, Fu YS, Selch MT, Parker RG (1994) Postirradiation sarcomas. A single-institution study and review of the literature. Cancer 73 (10): 2653–2662.
Marquardt H, Philips FS, Sternberg SS (1976) Tumorigenicity in vivo and induction of malignant transformation and mutagenesis in cell cultures by adriamycin and daunomycin. Cancer Res 36 (6): 2065–2069.
Martland H (1929) Occupational poisoning In manufacture of luminous watch dials. J Am Med Assoc 92 (7): 552–559.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA: Cancer J Clin 66 (4): 271–289.
Moppett J, Oakhill A, Duncan AW (2001) Second malignancies in children: the usual suspects? Eur J Radiol 38 (3): 235–248.
Newton WA Jr, Meadows AT, Shimada H, Bunin GR, Vawter GF (1991) Bone sarcomas as second malignant neoplasms following childhood cancer. Cancer 67 (1): 193–201.
Nottage K, McFarlane J, Krasin MJ, Li C, Srivastava D, Robison LL, Hudson MM (2012) Secondary colorectal carcinoma after childhood cancer. J Clin Oncol 30 (20): 2552–2558.
Schaapveld M, Aleman BM, van Eggermond AM, Janus CP, Krol AD, van der Maazen RW, Roesink J, Raemaekers JM, de Boer JP, Zijlstra JM, van Imhoff GW, Petersen EJ, Poortmans PM, Beijert M, Lybeert ML, Mulder I, Visser O, Louwman MW, Krul IM, Lugtenburg PJ, van Leeuwen FE (2015) Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med 373 (26): 2499–2511.
Schneider K, Zelley K, Nichols KE, Garber J (1993) Li-Fraumeni syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K (eds). Gene Reviews (R). University of Washington (Internet): Seattle, WA, USA.
Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE (2012) Radiation-induced sarcoma of the breast: a systematic review. Oncologist 17 (3): 405–418.
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62 (4): 220–241.
Suttie CF, Hong A, Stalley P, Veillard AS, Tattersall MH (2012) Does chemotherapy shorten the latency interval of radiation-induced sarcomas? Clin Oncol (R Coll Radiol) 24 (1): 77–79.
Swerdlow AJ, Schoemaker MJ, Allerton R, Horwich A, Barber JA, Cunningham D, Lister TA, Rohatiner AZ, Vaughan Hudson G, Williams MV, Linch DC (2001) Lung cancer after Hodgkin's disease: a nested case-control study of the relation to treatment. J Clin Oncol 19 (6): 1610–1618.
Thijssens KM, van Ginkel RJ, Suurmeijer AJ, Pras E, van der Graaf WT, Hollander M, Hoekstra HJ (2005) Radiation-induced sarcoma: a challenge for the surgeon. Ann Surg Oncol 12 (3): 237–245.
Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E, Storm H, Glimelius I, Pukkala E, Stovall M, Fraumeni JF Jr, Boice JD Jr, Gilbert E (2002) Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst 94 (3): 182–192.
Trip AK, Stiekema J, Visser O, Dikken JL, Cats A, Boot H, van Sandick JW, Jansen EP, Verheij M (2015) Recent trends and predictors of multimodality treatment for oesophageal, oesophagogastric junction, and gastric cancer: a Dutch cohort-study. Acta Oncol 54 (10): 1754–1762.
Tucker MA, D’Angio GJ, Boice JD Jr, Strong LC, Li FP, Stovall M, Stone BJ, Green DM, Lombardi F, Newton W et al (1987) Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 317 (10): 588–593.
Westendorf J, Marquardt H, Ketkar MB, Mohr U, Marquardt H (1983) Tumorigenicity in vivo and induction of mutagenesis and DNA repair in vitro by aclacinomycin A and marcellomycin: structure-activity relationship and predictive value of short-term tests. Cancer Res 43 (11): 5248–5251.
Acknowledgements
This work was supported by the European Society for Medical Oncology Clinical Unit Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Zhang, A., Judson, I., Benson, C. et al. Chemotherapy with radiotherapy influences time-to-development of radiation-induced sarcomas: a multicenter study. Br J Cancer 117, 326–331 (2017). https://doi.org/10.1038/bjc.2017.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.198