Abstract
Previous trials showed the tolerability and efficacy of a palliative radiotherapy (RT) regimen (SHARON) based on the 4 fractions delivered in 2 days in different oncological settings. In order to identify possible predictors of symptomatic response, the purpose of this study is to perform a pooled analysis of previous trials. We analyzed the impact on symptomatic response of the following parameters: tumor site, histological type, performance status (ECOG), dominant symptom, and RT dose using the Chi-square test and Fisher’s exact test. One-hundred-eighty patients were analyzed. Median RT dose was 20 Gy (range: 14–20 Gy). The overall response rate was 88.8% (95% CI 83.3–92.7%) while pre- and post-treatment mean VAS was 5.3 (± 7.7) and 2.2 (± 2.2), respectively (p < 0.001). The overall response rate of pain, dyspnea, bleeding, dysphagia, and other symptoms was 86.2%, 90.9%, 100%, 87.5%, and 100%, respectively. Comparing the symptomatic effect based on the analyzed parameters no significant differences were recorded. However, patients with locally advanced disease showed a higher rate of symptomatic responses than metastatic ones (97.3% vs 83.0%; p = 0.021). Finally, the complete pain response rate was more than double in patients with mild to moderate (VAS: 4–7) compared to those with severe (VAS > 7) pain (36.0% vs 14.3%; p = 0.028). This pooled analysis showed high efficacy of the SHARON regimen in the relief of several cancer-related symptoms. The markedly and significantly higher complete pain response rate, in patients with mild-moderate pain, suggests early referral to palliative RT for patients with cancer-related pain.
Similar content being viewed by others
Introduction
Radiation therapy (RT) has a marked effect on tumor-related symptoms. Therefore, palliative RT is frequently used in the treatment of symptomatic patients with advanced cancer.
Short treatments are preferable in the setting of palliative RT. In fact, the poor patients’ clinical conditions limit the use of prolonged therapies. In addition, the symptomatic relief from even low RT doses, and therefore the low risk of toxicity, promotes the use of accelerated treatments delivered in few fractions (accelerated-hypofractionated RT). Finally, the treatment acceleration is theoretically associated with a more rapid tumor response and therefore with better and faster palliative response.
Some studies tested regimens based on four fractions administered in two days in the palliative treatment of advanced tumors of the uterine cervix1,2 and of the head and neck3,4,5. These trials showed good tolerability and symptomatic efficacy using doses of approximately 14–15 Gy in two days and repeating these treatments up to three times 2–4 weeks apart.
We tested a similar regimen (SHARON: SHort-course Accelerated radiatiON therapy) but in a single cycle, in the same and other settings (multiple brain and complicated bone metastases, advanced tumors of head-neck, thorax, and pelvis). A first series of phase I trials demonstrated the feasibility, using 3D-conformal RT, of administering 18–20 Gy in two days6,7. Furthermore, subsequent phase II trials recorded consistently higher than 80% response rates in the settings mentioned above8,9.
However, our studies evaluated the SHARON regimen in single settings and in small patients series and therefore some questions remain open. In particular, it is not known whether SHARON is equally effective regardless of the anatomical site of the treated lesion, histological type, patient's performance status, and the dominant symptom. Furthermore, the impact of moderate doses (< 18 Gy) versus high doses (18–20 Gy) on symptom relief was not previously analyzed. Therefore, the purpose of this study is to perform a pooled analysis of the previous trials, on a large patients population, in order to clarify these topics.
Materials and methods
Study design and end points
This is a retrospective pooled analysis of symptomatic response in patients with locally advanced or metastatic tumors undergoing the SHARON regimen and enrolled in prospective phase I and II trials. The methods used for RT planning and delivery, patient evaluation and data analysis were previously described6,7,8,9 and will only be summarized here.
Inclusion criteria
Patients with symptomatic locally advanced or metastatic solid tumors were included in the SHARON trials. Patients with ECOG performance status > 3, pregnant, aged < 18 years, and previously irradiated in the same anatomic site were excluded.
Radiotherapy planning and delivery
RT was planned with a CT-simulation in the treatment position. The gross tumor volume (GTV) was defined as the symptomatic macroscopic lesion. The clinical target volume (CTV) was defined as the GTV with the addition of 1.0 cm isotropic margin while the planning target volume was defined as the CTV plus an additional 1.0 cm isotropic margin. The latter margin was 1.5 cm in the cranial-caudal direction in patients with thoracic or upper abdomen tumors. The dose prescription and specification was based on the ICRU 62 report10. The two daily RT fractions were delivered with an interval of 6 h. However, in patients with spinal cord irradiation, the interval was extended to 8 h. Before each fraction, a set-up check was performed using an electronic portal imaging device as previously described11.
Follow up
Patient evaluation based on physical examination and complete blood count, was performed 15 days after RT. During the same visit we evaluated both symptomatic response and quality of life using the Cancer Linear Analogue Scales (CLAS), a tool based on an analog-visual scale, with values from 0 to 10, measuring the level of well-being (CLAS1), fatigue (CLAS2), and the ability to carry out daily activities (CLAS3)12. Subsequently, patients were evaluated every two months by recording symptoms’ control and RT-related toxicity. Further instrumental tests were required based on the physician's clinical judgment.
Clinical and statistical issues
Pain was scored both using a Visual Analogue self-assessment Scale (VAS) and according to the International Atomic Energy Agency criteria, using the pain score and drug score parameters13. Pain response was graded according to International Bone Metastases Consensus Working Party criteria14. For other symptoms, the patient's reported reduction or complete disappearance of symptoms was defined as partial and complete response, respectively. Furthermore, the complete suspension of ongoing therapies for the specific symptom was required to define a complete response. The overall response rate was defined as the sum of complete and partial responses. The symptomatic progression-free actuarial survival curve during follow-up was calculated with the Kaplan–Meier method. Toxicity was scored according to the Radiation Therapy Oncology Group (RTOG) and the RTOG/European Organization for Research and Treatment of Cancer criteria for acute and late adverse events, respectively15. We used the chi-square test of independence, or Fisher’s Exact test for contingency tables in case of a cell-count of less than 5, to analyze the impact of the considered categorical parameters on symptomatic response. Statistical analyses were performed using the IBM SPSS Statistic version 20 software package.
Ethical issues
All trials on which this analysis is based were approved by the Ethics Committees of the institution in which the enrollment and treatment were performed (Gemelli Molise Hospital, Campobasso, Italy). All experiments were performed in accordance with relevant guidelines and regulations. All patients signed a written consent to their inclusion in the trials before RT planning.
Ethics approval and consent to participate
The study was approved by the local Ethics Committee. All patients provided written informed consent before the study entry.
Results
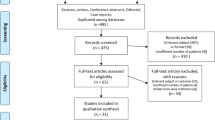
One hundred and eighty patients were included in this pooled analysis (M/F: 100/80; median age: 70 years; range: 39–98 years; median follow-up: 5 months; range: 1–36 months). Other characteristics of the subjects included in this study are shown in Table 1. No patient died before the evaluation 15 days after radiotherapy, and all patients were examined at the first follow-up after treatment (15 days). Instead, at the first visit of follow-up (2 months) the rate of patients who died or were not evaluable, due to worsening of the general condition that precluded the visit, was 6% and 3%, respectively. Moreover, the rate of patients who could be evaluated at 6 and 12 months was 50% and 25%, respectively (Fig. 1).
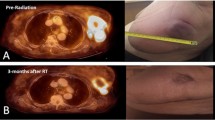
Figure 2 shows the radiological response of an advanced non-small cell lung tumor after palliative RT. A complete or partial symptomatic response was recorded in 51 (28.3%) and 109 (60.5%) patients, respectively, with 88.8% (95% CI 83.3–92.7%) overall response rate and four months median time to symptoms progression. The pre- and post-treatment mean VAS was 5.3 (± 7.7) and 2.2 (± 2.2) respectively (p < 0.00001). At first follow-up, 76 (42.2%), 84 (46.7%), and 20 (11.1%) patients showed improved, stable, and worse ECOG performance status, respectively. An improvement of CLAS 1, 2, and 3 scores was recorded in 80 (48.2%), 70 (42.2%), and 77 (46.4%) out of 167 evaluable patients, respectively. Four patients (2.2%) presented Grade ≥ 3 acute toxicity while one patient (0.6%) showed Grade 3 late toxicity. Comparing the symptomatic effect of the SHARON regimen between anatomical sites, histological types, ECOG scores and RT doses (< 18 vs ≥ 18 Gy) no significant differences were recorded (data not shown). Similarly, the response rate of the different symptoms was similar, without significant differences (data not shown). In contrast, patients with locally advanced disease showed a higher rate of symptomatic responses than metastatic ones (97.3% vs 83.0%; p = 0.021). Finally, the complete pain response rate was more than double in patients with mild to moderate (VAS: 4–7) pain compared to those with severe (VAS > 7) pain (36.0% vs 14.3%; p = 0.028).
Discussion
With 88.8% overall symptomatic response rate and only five patients (2.8%) with severe toxicity, this pooled analysis confirmed the tolerability and efficacy of the SHARON regimen in the relief of cancer-related symptoms. In particular, the analysis showed a symptomatic effect independent of tumor site, histological type, prevalent symptom, and delivered RT dose. However, even without statistically significant differences, it can be noted that bleeding showed the highest overall response rate (100%) and that squamous cell tumors showed a slightly higher response rate than adenocarcinomas (92.0% vs 87.8%). Furthermore, our analysis did not show a significant impact of RT dose on symptom relief. This result may be important because it suggests the use of this regimen (with possibly reduced doses) even in settings where advanced or simply conformal RT techniques are not available or in case of reirradiation.
In contrast, a higher response rate of the SHARON regimen was observed in patients with locally advanced disease than in those with metastatic tumors. It is not easy to explain this difference. It can be assumed that metastatic patients have generally multiple lesions with difficult registration of the symptomatic effect in the irradiated lesion alone.
Furthermore, our analysis showed a significantly higher complete pain response rate in patients with mild to moderate pain compared to patients with severe pain. This result suggests that palliative RT is less effective in terms of pain relief in patients with more severe symptoms, as confirmed by a previous analysis showing a higher rate of pain palliation in patients not receiving opioid therapy16. Furthermore, based on another analysis showing that long-lasting oncologic pain is significantly worse17, it can be hypothesized that early palliative RT may be more effective than RT delivered to subjects with more prolonged pain. This hypothesis would be in line with a further recent analysis showing that patients treated with palliative RT and with shorter pain duration (< 1 month) had higher cumulative incidence of pain response (subdistribution hazard ratio, 2.43; 95% CI 1.35–4.38) compared with subjects with longer pain duration (≥ 4 months)18. However, we should also consider that the higher efficacy of early palliative RT could derive from a milder pain in the initial stages of its onset but also from the well-known refractoriness of long-lasting and therefore chronic pain.
As anticipated, previous studies tested regimens based on four fractions in two days, with a total dose lower than ours but repeating RT up to three consecutive cycles. It is not possible to compare their results with those of our analysis because they used accelerated regimens in single tumor settings (mainly cervix and head and neck)3,4,5. However, the uniformly high response rates we recorded with only one RT course cast doubt on the need to treat all patients with repeated cycles. In fact, an alternative and more convenient strategy could be based on the delivery of a single course in patients with satisfactory symptomatic response and reserving repeated treatments only for non-responders.
This analysis has obvious limitations. A relatively basic Patients Reported Outcome Measure (CLAS scale) was used to assess quality of life. Furthermore, the retrospective design of the study is an additional limitation, even if the patients were enrolled, treated, and analyzed prospectively.
Despite these limitations, some clinical conclusions can be drawn. In fact, based on its tolerability and efficacy, the SHARON regimen can be proposed in clinical practice especially in: (i) patients residing far from the RT center; to minimize the need for travel and overnight stays, (ii) busy RT departments, in order to reduce waiting lists and to allow prompt treatment of symptoms, (iii) in situations such as the current SARS-CoV-2 pandemic, where it is preferable to limit the stay of cancer patients in the hospital environment due to the risk of infection.
Moreover, these results justify the design of further studies to improve the effectiveness of this regimen. In fact, despite uniformly high overall response rates, the complete symptomatic response rate was < 50% in all analyzed subgroups. Therefore, new trials could aim to: (i) improve the current results in terms of tumor response with the use of innovative irradiation modalities such as Partially Ablative RT (PART)19 and techniques based on the use of microbeams (GRID, lattice)20; (ii) further improve the toxicity profile through the use of modulated RT techniques5; (iii) optimize the integration between palliative RT and pharmacological palliative care; (iv) test the SHARON regimen in developing countries, where this short treatment would be particularly useful given the shortcomings in terms of RT centers and equipment21.
In conclusion, this pooled analysis showed the symptomatic efficacy of the SHARON regimen independent of anatomic site, histological type, performance status, prevalent symptom, and delivered RT dose. Seven randomized trials are currently underway in our centers to compare the results of this accelerated treatment (20 Gy in two days) with those of a traditional palliative RT treatment (30 Gy in 2 weeks) in different tumor settings (NCT03804307, NCT03804333, NCT03503682).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Spanos, W. J. Jr. et al. Late effect of multiple daily fraction palliation schedule for advanced pelvic malignancies (RTOG 8502). Int. J. Radiat. Oncol. Biol. Phys. 30(29), 961–967 (1994).
Spanos, W. Jr. et al. Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: preliminary report of RTOG 8502. Int. J. Radiat. Oncol. Biol. Phys. 17, 659–661 (1989).
Corry, J. et al. The “QUAD SHOT”: a Phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother. Oncol. 77, 137–142 (2005).
Lok, B. H. et al. Palliative head and neck radiotherapy with the RTOG 8502 regimen for incurable primary or metastatic cancers. Oral Oncol. 51, 957–962 (2015).
Toya, R. et al. Hypofractionated palliative volumetric modulated arc radiotherapy with the Radiation Oncology Study Group 8502 “QUAD shot” regimen for incurable head and neck cancer. Radiat. Oncol. 15, 123 (2020).
Caravatta, L. et al. Short-course accelerated radiotherapy in palliative treatment of advanced pelvic malignancies: a Phase I study. Int. J. Radiat. Oncol. Biol. Phys. 83, e627–e631 (2012).
Farina, E. et al. Phase I-II study of short-course accelerated radiotherapy (SHARON) for palliation in head and neck cancer. Anticancer Res. 38, 2409–2414 (2018).
Farina, E. et al. Palliative short-course radiotherapy in advanced pelvic cancer: a phase II study (SHARON Project). Anticancer Res. 39, 4237–4242 (2019).
Capuccini, J. et al. Short-course regimen of palliative radiotherapy in complicated bone metastases: a Phase I-II study (SHARON Project). Clin. Exp. Metastasis. 35, 605–611 (2018).
Morgan-Fletcher, S. L. Prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50), ICRU Report 62. ICRU, pp ix + 52, 1999 (ICRU Bethesda, MD). https://www.icru.org/report/prescribing-recording-and-reporting-photon-beam-therapy-report-62/ (1999).
Deodato, F. et al. Daily on-line set-up correction in 3D-conformal radiotherapy: is it feasible?. Tumori 98, 441–444 (2012).
Sutherland, H. J., Walker, P. & Til, J. E. The development of a method for determining oncology patients’ emotional distress using linear analogue scales. Cancer Nurs. 11(5), 303–308 (1988).
Salazar, O. M. et al. Fractionated half-body irradiation (HBI) for the rapid palliation of widespread, symptomatic, metastatic bone disease: a randomized Phase III trial of the International Atomic Energy Agency (IAEA). Int. J. Radiat. Oncol. Biol. Phys. 50, 765–775 (2001).
Chow, E. et al. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 82, 1730–1737 (2012).
Cox, J. D., Stetz, J. & Pajak, T. F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J Radiat. Oncol. Biol. Phys. 31, 1341–1346 (1995).
Saito, T. et al. Predictors of pain palliation after radiation therapy for painful tumors: a prospective observational study. Int. J. Radiat. Oncol. Biol. Phys. 101, 1061–1068 (2018).
Shin, J. et al. Distinct worst pain profiles in oncology outpatients undergoing chemotherapy. Cancer Nurs. 10, 1097 (2022).
Saito, T. et al. Influence of pain duration on pain outcomes following palliative radiotherapy for painful tumors: the sooner the irradiation, the better?. Strahlenther. Onkol. 197, 916–925 (2021).
Cilla, S. et al. Partially ablative radiotherapy (PAR) for large mass tumors using simultaneous integrated boost: a dose-escalation feasibility study. J. Appl. Clin. Med. Phys. 19, 35–43 (2018).
Fukunaga, H., Butterworth, K. T., McMahon, S. J. & Prise, K. M. A brief overview of the preclinical and clinical radiobiology of microbeam radiotherapy. Clin. Oncol. (R Coll. Radiol.) 33, 705–712 (2021).
Deressa, B. T. et al. Short-course 2-dimensional radiation therapy in the palliative treatment of esophageal cancer in a developing country: a Phase II study (Sharon Project). Int. J. Radiat. Oncol. Biol. Phys. 106, 67–72 (2020).
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conception and design: all authors. Research and data collection: G.M., F.D., S.C., L.C., and A.G.M.; Analysis and interpretation of data: C.M.D., G.S., A.Z., M.B., S.C., and A.G.M.; Manuscript writing: all authors. Approval of final article: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donati, C.M., Macchia, G., Siepe, G. et al. Short course palliative radiotherapy in advanced solid tumors: a pooled analysis (the SHARON project). Sci Rep 12, 20978 (2022). https://doi.org/10.1038/s41598-022-25602-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25602-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.