Abstract
Background:
The prevalence of breast lesions (benign, precancerous and cancer lesions) in reduction mammaplasty (RM) specimens has rarely been reported in Europe and never in the Swiss population.
Methods:
Personal and histopathological data from 534 female patients who underwent RM were reviewed.
Results:
Benign and/or malignant lesions were detected in 76.2% of all patients. Benign breast lesions associated with an increased risk of developing breast cancer represented 2.8% of all lesions. Breast cancer in situ was identified in 5 (0.9%) patients. Patient age and previous history of breast cancer were risk factors for incidental breast cancer.
Conclusion:
The rate of incidental carcinoma in situ was higher for patients with breast cancer history. Probably due to preoperative breast cancer investigation, no occult invasive breast cancer was found in reduction mammary specimens. Therefore before RM, breast cancer evaluation should be considered for all patients, especially for those with breast cancer risk factors (e.g., patient age, personal history of breast cancer).
Similar content being viewed by others
Main
Reduction mammaplasty (RM) is one of the most common plastic surgery procedures. An estimated 35%–60% of all patients undergoing RM are ⩾40 years (Ishag et al, 2003), when the incidence rate of breast cancer begins to rise. The occurrence of incidental breast cancer in RM specimens is therefore not surprising, and several studies have shown its prevalence to range from 0.06% to 3.8% (Tang et al, 1999; Ishag et al, 2003; Cook and Fuller 2004; Ambaye et al, 2009; Clark et al, 2009). Moreover, several benign breast lesions are associated with an increased risk of subsequent breast cancer (Fitzgibbons et al, 1998; Shaaban et al, 2002; Wang et al, 2004; Hartmann et al, 2005). The prevalence of incidental breast lesions in RM specimens in the Swiss population has not been reported. This population is of particular interest, because their incidence of breast cancer is one of the highest in Europe (Bouchardy et al, 2006).
In this study, we aimed to determine the prevalence of both benign and malignant breast lesions in a series of 534 consecutive patients who underwent RM at a single Swiss academic center after negative breast cancer evaluation. We attempted to identify risk factors for incidental breast cancer, such as specimen weight, patient age and previous history of breast cancer. In addition, we compared the prevalence of breast cancer among our RM patients with the prevalence expected in a comparable local population, using local Cancer Registry data.
Patients and methods
Patients
Medical charts of 534 consecutive female patients who underwent RM between February 1990 and November 2010 at our Plastic, Reconstructive and Aesthetic Surgery Department were retrospectively reviewed from a prospectively maintained database. All women were clinically assessed by a breast specialist before RM. Patients aged ⩾50 years were further evaluated by mammogram according to the Swiss and European guidelines for breast cancer screening (Wald et al, 1993; Huwiler 2010). Younger patients with positive personal or family history of breast cancer or with abnormal breast examination were evaluated by mammogram, breast ultrasound or magnetic resonance imaging (MRI) at the discretion of the treating physician. Patients with negative breast examination and no suspicious findings in radiological studies were eligible for RM. All operations were performed by staff plastic surgeons using the standard superior pedicle technique. The study protocol was reviewed and approved by the local Clinical Ethics.
Pathological examination
After an overnight formalin fixation, a gross examination was performed on 1 cm-thick sections. When no lump was palpable or macroscopically evident, a tissue sampling was randomly done. Between two and four samples per breast were taken from each resected breast specimen. After paraffin embedding, 4–5 μ-thick sections were cut and stained with hematoxylin and eosin. When breast cancer was microscopically detected, additional samples were taken and surgical margins were assessed.
Variables of interest
Personal history of breast cancer, age at RM, weight and histopathological findings of RM specimens were available. The classification used to categorise histopathological findings was adapted from Guray and Sahin (2006). For those patients with incidental breast cancer, clinical management and follow-up history are described.
Statistics
We established the prevalence of breast lesions in the cohort and performed stratification by breast cancer history (yes vs no) and patient age (⩽50 vs >50 years) and specimen weight (⩽500 vs >500 g). Differences in clinical and pathological variables between the different groups of patients were assessed with the χ2-test for categorical variables and Student’s t-test for continuous variables, using commercially available software. A two-sided P value<0.05 was considered significant.
To compare the incidence of breast cancer between our cohort and the expected rate in the general local female population, we calculated standardised incidence ratios (SIRs). The expected number of in situ and invasive breast cancers was calculated by multiplying the calculated person-years at risk (stratified by 5-year intervals of age and calendar years) by the specific in situ and invasive breast cancer incidence rates of the local female population stratified by 5-year intervals of age and calendar period. We then calculated SIR as the ratio of the observed events in the cohort to the expected number of events in the general population. We calculated SIRs according to the presence or not of the previous history of breast cancer. Calculations of SIRs were done with PYRS software (Coleman et al, 1989). All P-values are two-sided and calculated by Fisher’s exact test.
Results
Among the 534 patients, 479 (89.7%) were operated on for symptomatic mammary hypertrophy without previous personal breast cancer history and 55 (10.3%) for breast asymmetry after contralateral breast cancer surgery.
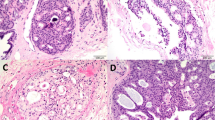
Overall, benign and/or malignant histopathological findings were observed in 76.2% of 534 patients (Table 1) and the prevalence was similar between patients without and with previous history of breast cancer (P=0.52). Fibrocystic changes were the most common lesions (73%) (details in Supplementary Material online). Microcalcifications were found in 19 (3.6%) patients, all without concomitant breast cancer, and were significantly associated with previous history of breast cancer and patients >50 years.
Benign breast neoplasms were detected in 21 (3.9%) patients, all of whom had no previous history of breast cancer: majority was fibroadenomas (3%) and some few cases lipomas, adenomas, nipple adenomas and hamartomas.
Benign breast lesions associated with an increased risk of developing subsequent breast cancer according to the Cancer Committee of the College of American Pathologists (Fitzgibbons et al, 1998) (e.g., sclerosing adenosis, moderate and atypical epithelial hyperplasia, intraductal papilloma and papillomatosis) represented 2.8% of all lesions. (online Supplementary Data).
Incidental breast cancer was identified in 5 (0.9%) patients, including 2 cases of ductal and 3 cases of lobular carcinoma in situ. No invasive cancer was found. Median age of women with incidental cancer found at the time of RM was higher than those without cancer lesions (50 vs 38 years, P<0.001). The in situ cancer prevalence was significantly higher in patients with previous contralateral breast cancer in comparison to women without previous history of breast cancer (5.5% vs 0.4%, P=0.009).
A single patient had multifocal ductal in situ carcinoma and underwent immediate radiotherapy after RM. She had local recurrence of a ductal carcinoma in situ 6 months after radiotherapy and underwent radical mastectomy with axillary lymph node dissection. The other four patients were closely followed up (Table 2). The survival rate was 100% for the five patients after a median follow-up of 89 (range 56–136) months.
The number of in situ breast cancer among our cohort of women undergoing RM was higher than the number of cases expected among the general local population (observed in situ cancer=5, expected in situ cancer=0.81, SIR=6.2; 95% confidence interval (CI)=2.0–14.4, P=0.002). This risk excess was accentuated for patients who had previous history of breast cancer, compared with the local population with previous history of breast cancer (observed in situ cancer=3, expected in situ cancer=0.23, SIR=13.1; 95% CI=2.7–38.1, P=0.002). There was no significant difference for patients without previous history of breast cancer (observed in situ cancer=2, expected in situ cancer=0.58, SIR=3.4; 95% CI=0.4–12.5, P=0.23). In addition, although the expected number of invasive breast cancer was 7.93, no invasive breast cancer was detected.
Discussion
In a cohort of 534 consecutive patients who underwent RM after negative breast cancer evaluation, we found that 76.2% of patients presented a benign histopathological breast lesions. Some benign lesions are known to be associated with increased risk to develop breast cancer in the future. Hartmann et al (2005) found a relative risk for breast cancer of 4.2 associated with atypia lesions, 1.9 for proliferative changes without atypia and 1.3 for non-proliferative lesions. Moreover, Wang et al (2004) demonstrated that the risk of breast cancer was significantly increased even for low-risk benign breast lesions, independently from other risk factors for breast cancer. Even microcalcifications alone on biopsy specimens have a slightly higher relative risk (1.41) of subsequent breast cancer (Shaaban et al, 2002). Assessing only the benign lesions associated with a high risk of breast cancer according to the American College of Pathologists, we demonstrated that 2.8% of our patients had this type of lesions in their RM specimen. Two studies from the United States reported even higher prevalence rates (5.5% (Blansfield et al, 2004) and 10.7% (Ishag et al, 2003)). Therefore, we believe that reporting these findings should not be overlooked. A tailored follow-up strategy should then be offered to patients diagnosed with at-risk lesions and involve an interdisciplinary team approach.
In our study, the prevalence of in situ breast cancer discovered incidentally in RM specimens was 0.9%, and significantly higher for patients with previous history of contralateral breast cancer than for patients without previous breast cancer history (5.5% vs 0.4%, P=0.009). This prevalence for patients with previous contralateral breast cancer is in the range of previous studies (Slezak and Bluebond-Langner, 2011: 1.3%; Clark et al, 2009: 6%; Freedman et al, 2012: 7.1%); but 13 times more compared with the expected number in a comparable female local population with previous history of breast cancer. Our data confirm that for patients with previous history of breast cancer who undergo RM, a higher risk of incidental breast cancer exists, especially in older patients. Therefore, more advanced imaging such as MRI during preoperative evaluation and/or more intense pathological workup may be justified in selected patients.
Concerning invasive breast cancer, although 7.93 cases of invasive breast cancer were expected in a comparable local general population, we did not identify any case. This is concordant with other studies that reported low frequency rates of invasive breast cancer ranging from 0% to 0.9% in RM specimens (Cook and Fuller, 2004; Ambaye et al, 2009; Clark et al, 2009; Slezak and Bluebond-Langner, 2011). As invasive cancer is more likely to be detected during clinical examination and on imaging studies than in situ lesions, the absence of invasive lesions might reflect an effective preoperative patient assessment.
On diagnosis of breast cancer in RM specimens, a varying range of management has been reported, including observation only, tamoxifen alone (Cook and Fuller, 2004), completion mastectomy, radiation therapy (Clark et al, 2009) or a combination of radiation therapy and tamoxifen (Ishag et al, 2003).
In conclusion, for every four patients who underwent a RM, three of them presented a present breast lesions, mostly benign but some with cancer lesions and others with lesions associated with increased risk of subsequent breast cancer. Therefore for patients undergoing RM, breast cancer assessment, including information on previous history of breast cancer, clinical examination, and, if indicated, radiological studies, remain of utmost importance. Particularly for patients with past history of breast cancer and older women who present a higher risk of incidental breast cancer, more advanced imaging such as preoperative MRI and more intense pathological workup may be justified. These measures may decrease the rate of incidental invasive breast cancer. Whether these would impact patient management and be cost-effective remains to be elucidated. Therefore, further efforts in the form of standardised guidelines should be made to optimise collaboration between plastic surgeons, oncologists, pathologists and radiologists.
Change history
04 February 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ambaye AB, Mac Lennan SE, Goodwin AJ, Suppan T, Naud S, Weaver DL (2009) Carcinoma and atypical hyperplasia in reduction mammaplasty: increased sampling leads to increased detection. A prospective study. Plast Reconstr Surg 124: 1386–1392.
Blansfield JA, Kukora JS, Goldhahn Jr RT, Buinewicz BR (2004) Suspicious findings in reduction mammoplasty specimens: review of 182 consecutive patients. Ann Plast Surg 52: 126–130.
Bouchardy C, Morabia A, Verkooijen HM, Fioretta G, Wespi Y, Schäfer P (2006) Remarkable change in age-specific breast cancer incidence in the Swiss Canton of Geneva an its possible relation with the use of hormone replacement therapy. BMC Cancer 6: 78–85.
Clark CJ, Whang S, Paige KT (2009) Incidence of precancerous lesions in breast reduction tissue: a pathologic review of 562 consecutive patients. Plast Reconstr Surg 124: 1033–1039.
Coleman MP, Hermon C, Douglas A (1989) Person-Years (PYRS). A Fortran Program for Cohort Study Analysis. International Agency for Research on Cancer (IARC) Internal Report No. 89/006. IARC: Lyon, France.
Cook IS, Fuller CE (2004) Does histopathological examination of breast reduction specimens affect patient management and clinical follow up? J Clin Pathol 57: 286–289.
Fitzgibbons PL, Henson DE, Hutter RVP (1998) Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med 122: 1053–1055.
Freedman BC, Rosenbaum Smith SM, Estabrook A, Balderacchi J, Tartter PI (2012) Incidence of occult carcinoma and high-risk lesions in mammoplasty specimens. Int J Breast Cancer 2012: 145630.
Guray M, Sahin AA (2006) Benign breast diseases: classification, diagnosis and management. Oncologist 11: 435–449.
Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton 3rd LJ, Visscher DW (2005) Benign breast disease and the risk of breast cancer. N Engl J Med 353: 229–237.
Huwiler K (2010) Screening mammography: answer to essential questions. The Swiss Cancer League Available at http://www.liguecancer.ch (accessed 1 June 2013).
Ishag MT, Baschinsky DY, Beliaeva IV, Niemann TH, Marsh Jr WL (2003) Pathologic findings in reduction mammaplasty specimens. Am J Clin Pathol 120: 377–380.
Shaaban AM, Sloane JP, West CR, Moore FR, Jarvis C, Williams EM, Foster CS (2002) Histopathologic types of benign breast lesions and the risk of breast cancer: case-control study. Am J Surg Pathol 26: 421–430.
Slezak S, Bluebond-Langner R (2011) Occult carcinoma in 866 reduction mammaplasties: preserving the choice of lumpectomy. Plast Reconstr Surg 127: 525–530.
Tang CL, Brown MH, Levine R, Sloan M, Chong N, Holowaty E (1999) Breast cancer found at the time of breast reduction. Plast Reconstr Surg 103: 1682–1686.
Wald NJ, Chamberlain J, Hackshaw A on behalf of the Evaluation Committee (1993) Report of the European Society of Mastology Breast Cancer Screening Evaluation Committee. Breast 2: 209–216.
Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N (2004) Lower-category benign breast disease and the risk of invasive breast cancer. J Natl Cancer Inst 96: 616–620.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tadler, M., Vlastos, G., Pelte, MF. et al. Breast lesions in reduction mammaplasty specimens: a histopathological pattern in 534 patients. Br J Cancer 110, 788–791 (2014). https://doi.org/10.1038/bjc.2013.708
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.708