Abstract
Background:
Allogeneic haematopoietic stem cell transplantation (allo-SCT) may provide donor cytotoxic T cell-/NK cell-mediated disease control in patients with rhabdomyosarcoma (RMS). However, little is known about the prevalence of graft-vs-RMS effects and only a few case experiences have been reported.
Methods:
We evaluated allo-SCT outcomes of 30 European Group for Blood and Marrow Transplantation (EBMT)-registered patients with advanced RMS regarding toxicity, progression-free survival (PFS) and overall survival (OS) after allo-SCT. Twenty patients were conditioned with reduced intensity and ten with high-dose chemotherapy. Twenty-three patients were transplanted with HLA-matched and seven with HLA-mismatched grafts. Three patients additionally received donor lymphocyte infusions (DLIs). Median follow-up was 9 months.
Results:
Three-year OS was 20% (s.e.±8%) with a median survival time of 12 months. Cumulative risk of progression was 67% (s.e.±10%) and 11% (s.e.±6%) for death of complications. Thirteen patients developed acute graft-vs-host disease (GvHD) and five developed chronic GvHD. Eighteen patients died of disease and four of complications. Eight patients survived in complete remission (CR) (median: 44 months). No patients with residual disease before allo-SCT were converted to CR.
Conclusion:
The use of allo-SCT in patients with advanced RMS is currently experimental. In a subset of patients, it may constitute a valuable approach for consolidating CR, but this needs to be validated in prospective trials.
Similar content being viewed by others
Main
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma (STS) in children and adolescents (Perez et al, 2011). As the term RMS describes a heterogeneous family of STS, histomorphology, tumour site, and clinical course may vary depending on the subtype. The most prevalent subtypes are embryonal RMS occurring in 67% and alveolar RMS occurring in ∼32% of RMS patients under the age of 20 years (Perez et al, 2011). Whereas embryonal RMS may harbour a broad spectrum of genetic aberrations, ∼80% of alveolar RMS are characterised by specific chromosomal translocations causing the fusion of the forkhead box O1 gene (FOXO1 alias FKHR) with either the paired box gene 3 (PAX3) or the PAX7 gene [t(2;13)(q35;q14) and t(1;13)(p36;q14)] leading to the formation of oncogenic transcription factors (Pappo et al, 1995). Although survival rates of patients with localised disease have considerably improved within past decades (Pappo et al, 1995; Stevens et al, 2005), metastatic and recurrent disease (advanced RMS) are commonly associated with fatal outcome (Stevens, 2005).
The implementation of high-dose chemotherapy (HDC) followed by autologous haematopoietic stem cell transplantation (SCT) could not achieve satisfactory overall survival (OS) rates in RMS patients (Koscielniak et al, 1997; Carli et al, 1999; Dantonello et al, 2009; Peinemann et al, 2011). Allogeneic haematopoietic SCT (allo-SCT) with or without the intentional infusion of donor lymphocytes (Tomblyn and Lazarus, 2008) has improved relapse-/progression-free survival (PFS) and OS in a growing number of high-risk patients with other cancer entities, possibly due to a T cell-/NK cell-mediated graft-vs-tumour effect (Childs et al, 2000; Ueno et al, 2003; Bishop et al, 2004; Bregni et al, 2004; Kolb et al, 2004; Lundqvist and Childs, 2005; Mackensen et al, 2006; Rizzo et al, 2009; Reisner et al, 2011). These observations suggest that allo-SCT and cellular immunotherapy may also improve outcome for RMS patients. However, little is known about graft-vs-RMS effects in patients treated with allo-SCT and only few single-centre case experiences have been reported (Misawa et al, 2003; Donker et al, 2009; Ohta et al, 2011).
In this retrospective study, we summarise the experiences drawn from the treatment of 30 patients with advanced RMS included in the European Group for Blood and Marrow Transplantation (EBMT) registries. All patients were treated with experimental allo-SCT and were not enrolled in ongoing prospective trials at the date of data censure. We evaluated their medical records in regard to conditioning regimens, HLA graft matching, toxicity, PFS, and OS to define the value of allo-SCT in the treatment of patients with advanced RMS and to discuss its potential in future immunotherapeutic approaches.
Patients and methods
Study design and data provenience
We evaluated data of all 30 EBMT-registered patients with advanced RMS and treated with allo-SCT between 1995 and 2011 (Tables 1, 2 and 3). Inclusion criteria were diagnosis of RMS (all subtypes), allo-SCT after 1995 and non-participation in ongoing prospective trials. Diagnosis was based on the clinical and histopathological examination. In nine patients with alveolar RMS, diagnoses were furthermore confirmed by molecular-genetic detection of specific chromosomal translocations. Three patients with alveolar RMS were translocation negative, whereas the presence of alveolar RMS was analysed merely histopathologically in 12 further patients (see also Table 2). Date of data censuring was 30 November 2011. In the following sections, patient numbers are followed by the indication of respective proportions given in brackets whenever appropriate.
Definitions
Engraftment was defined as an absolute neutrophil count of ⩾0.5 × 109 l−1 after allo-SCT. When patients died within 100 days post transplantation or when information was unavailable, chronic GvHD was considered as not assessable. Death of complications (DOCs) constituted any death occurring after allo-SCT in the absence of disease evidence including engraftment failure. The term death of disease (DOD) defines any death directly related to either disease progression or relapse. Progressive disease (PD) was defined as treatment-resistant increase in tumour volume, partial remission (PR) was defined as tumour volume reduction and complete remission (CR) as the absence of detectable disease. Residual disease included both PD and PR. Relapse-free survival (RFS) was defined as the time from last allo-SCT until the occurrence of any local or metastatic RMS evidence in patients who had reached CR after treatment. The PFS included RFS and was defined as the survival period after allo-SCT until date of relapse in patients transplanted in CR, and until date of progression diagnosis in case patients were transplanted with residual disease. Tumours were staged according to the WHO classification. HLA mismatch was defined as ⩾1 known allele mismatch in HLA class 1 and/or HLA class 2.
Statistical analyses
Data censure was conducted on 30 November 2011. Statistical analyses were performed using R 2.11.0 (The R Foundation for Statistical Computing, Vienna, Austria) and Prism 5 software (GraphPad Software, San Diego, CA, USA). Median survival time was defined as the time at which fractional survival equaled 50%. Time values for PFS and OS estimates were assessed starting on the date of the last allo-SCT until date of relapse/last follow-up and for OS until death independent of the cause or last follow-up. The PFS and OS probabilities were estimated using the Kaplan–Meier method with patients alive at last follow-up censored. Cumulative incidence curves were applied to estimate the occurrence of relapse and DOC, with DOC being a competing event for progression/relapse occurrence and vice versa as described (Scrucca et al, 2007). Standard errors (s.e.) for survival and cumulative risk estimates are given in brackets. As this is a retrospective study of a limited number of patients with heterogeneous clinical courses, statistical significance calculations regarding univariate group comparisons or multivariate analyses were not performed.
Results
Patient characteristics
All patients or their guardians gave written informed consent before therapy. Treatment relied on institutional review board approvals according to the Declaration of Helsinki. The study population consisted of 13 (43%) female and 17 (57%) male patients. Median age at diagnosis was 14 years (range: 2–28 years) and 16 years at allo-SCT (range: 4–28 years). Ten (33%) patients had received HDC and twenty (67%) patients reduced-intensity chemotherapy (RIC) before allo-SCT. In total, 23 (77%) patients received grafts from either HLA-matched related or matched unrelated donors, whereas 7 (23%) patients received either haplo-identical or otherwise HLA-mismatched grafts. Eligibility for allo-SCT was decided in case of relapse or PD after first-line treatment. Selection of patients suitable for allo-SCT was heterogeneous. In some of these patients, the presence of an HLA-matched sibling positively influenced the decision. After induction and conditioning treatment 24 patients received allografts in the absence of detectable disease after conditioning for allo-SCT, whereas 6 patients had residual disease after allo-SCT (Table 2). As this is a retrospective analysis of an internationally recruited study population, an objective side-by-side assessment by a single reference radiologist and reference pathologists was not performed. Graft source was bone marrow in 16 (53%) patients, peripheral blood in 10 (33%), and cord blood in 4 (13%) patients. Nine (3%) patients had received autologous grafts before allo-SCT. One patient received a second allogeneic graft due to initial graft failure. Three patients received donor lymphocyte infusions (DLIs) after allo-SCT. Patient characteristics are summarised in Table 1.
Conditioning regimen and GvHD prophylaxis
Reduced-intensity chemotherapy regimens were mainly based on fludarabine (FLU, 150–200 mg m−2) combined with the following drugs and/or total body irradiation (TBI): melphalan (MEL, 140 mg m−2; n=2), intravenous busulfan (BU, 6–8 mg kg−1; n=5), cyclophosphamide (CTX, 50–120 mg kg−1; n=1), CTX (50 mg kg−1) combined with 2 Gy TBI (n=4). In other patients, RIC comprised CTX (120 mg kg−1) with thiotepa (TT) (10 mg kg−1; n=2), MEL (140 mg m−2) combined with TT (15 mg kg−1; n=5) or TT alone (TT, unknown dosage; n=1).
High-dose chemotherapy comprised FLU (150 mg m−2) combined with treosulfan (TREO, 36 g m−2; n=1), CTX (120–180 mg kg−1) combined with oral BU (12.8 mg kg−1) and etoposide (ETO, 30 mg kg−1; n=2), MEL (140 mg m−2) combined with TT (10 mg kg−1) and carboplatin (CP, 1500 mg m−2; n=1), CP (unknown dosage) combined with TT (10 mg kg−1) and topotecan (TOPO, unknown dosage; n=1), FLU (120 mg m−2) combined with oral BU (16 mg kg−1) and TT (10 mg kg−1; n=1), FLU (150 mg m−2) combined with MEL (120 mg m−2) and TT (10 mg kg−1; n=1), CTX (180 mg kg−1) combined with oral BU (16 mg kg−1; N=1), CTX (120 mg kg−1) combined with oral BU (16 mg kg−1) and TT (10 mg kg−1; n=1) and FLU (150 mg m−2) combined with MEL (120 mg m−2) and TREO (36 g m−2, n=1). For assessment of conditioning regimens only the effect of the latest allo-SCT was analysed. The GvHD prophylaxis included methotrexate, mycophenolate-mofetil, tacrolimus, cyclosporine A, and/or prednisolone. At least one patient received OKT3 and at least seven patients received polyclonal anti-thymocyte globulins. Individual regimens are provided in Table 2.
Engraftment rates and GvHD
Twenty-seven (90%) patients engrafted successfully whereas three (10%) patients (patients #11, #20, and #24; Table 2) initially failed to engraft of whom one patient received a second allogeneic graft (patient #24; Table 2). Acute and chronic GvHD were defined in accordance with the ICD-10 system proposed by the WHO. Overall acute GvHD was reported in 13 (43%) patients. In 6 (20%) patients, chronic GvHD was not assessable due to either deathor last FU before day 100 after allo-SCT. Overall chronic GvHD occurred in 5 of 24 (21%) patients.
Within patients treated with HLA-matched grafts, 10 of 23 (43%) patients developed acute GvHD (I–II, n=4; III–IV, n=5; unavailable information in one patient). In the same group, 4 of 23 (17%) patients developed limited (n=3) or extensive (n=1) chronic GvHD, whereas status information remained unavailable in 4 of 23 patients due to early death or last follow-up before day 100 after allo-SCT. Within patients treated with mismatched grafts, 3 of 7 patients (43%) developed acute GvHD (I–II, n=2; III–IV, n=1), 1 of 7 (14%) patients developed extensive chronic GvHD and no patient developed limited chronic GvHD, whereas status information remained unavailable in 2 of 7 patients due to early death or last follow-up before day 100 after allo-SCT (Table 3).
Within patients treated with RIC as conditioning regimen for allo-SCT, 11 of 20 (55%) patients developed acute GvHD (I–II, n=5; III–IV, n=6). In the same group, 1 of 20 (5%) patients developed limited and no patient developed extensive chronic GvHD, whereas status information remained unavailable in 4 of 20 patients due to early death or last follow-up before day 100 after allo-SCT. Within patients treated with HDC as conditioning regimen for allo-SCT, 2 of 10 patients (20%) developed acute GvHD (III–IV, n=1; unavailable grade information in one patient), whereas 4 of 10 (40%) patients developed limited (n=2) chronic GvHD or extensive (n=2) chronic GvHD. Status information remained unavailable in 2 of 10 patients due to early death or last follow-up before day 100 after allo-SCT. In the whole group, one patient died due to GvHD (IV). Data summaries are given in Tables 2 and 3.
Overall survival
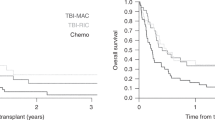
At the time of data censure, 22 of 30 (73%) patients had died due to disease or due to treatment-related complications and 8 of 30 (27%) patients were alive in CR (median: 44 months; range: 2–119 months). In all, 6 of 30 patients did not reach CR after allo-SCT. Median follow-up was 9 months (range: 1–119 months). Median survival time was 12 months. The OS estimate at day 100 after allo-SCT was 83% (s.e.±7%) and the 3-year OS estimate was 0.20 (s.e.±8%) (Figure 1). Survival data are summarised in Tables 2 and 3.
Progression-free survival
In total, 24 of 30 patients (80%) were in CR before allo-SCT, but none were converted from residual disease into CR. At data censure, 13 of 24 patients (54%) had relapsed, 3 (13%) patients had died due to complications in CR and 8 (33%) patients survived in CR (see above). One patient (patient #5; Table 2) died due to treatment-related complications after having relapsed. Median follow-up was 6 months (range: 1–119 months). The cumulative risk of disease progression including relapse for these patients was 34% (s.e.±9%) at day 100 and 67 (s.e.±10%) at 3 years after allo-SCT (Figure 2A). Results are summarised in Table 2.
(A) Cumulative risk analysis for progression of study group patients ( n= 30) after allo-SCT; patients #3, #9, #10, #15, #21, #23, #24, and #28 were alive at last follow-up were censored. (B) Cumulative risk analysis for treatment-related mortality in the study group (n=30) after allo-SCT; patients #3, #9, #10, #15, #21, #23, #24, and #28 were alive at last follow-up were censored. Abbreviations: DOC, death of complications; allo-SCT, allogeneic stem cell transplantation.
Death of complications
In all, 4 of 30 (13%) patients died due to treatment-related complications. The cumulative risk for DOC at day 100 after allo-SCT was 7% (s.e.±5%) and 11% (s.e.±6%) at 3 years after allo-SCT (Figure 2B). Reasons causing DOC were infection (n=2), veno-occlusive disease (n=1) and IV GvHD (n=1) (Tables 2 and 3).
Survival after reduced and high-dose chemotherapy
At data censuring, 1 of 20 (5%) patients treated with RIC had died due to treatment-related complications, 10 (50%) had relapsed and died, 4 (20%) had not reached CR and died and 5 (25%) patients were surviving in CR. Median follow-up in RIC-treated patients was 8 months (range: 1–62 months).
Of 10 patients treated with HDC-based conditioning 3 (33%) had died due to treatment-related complications, 3 patients (33%) relapsed (of whom 1 died of complications after relapse and was thus classified as both relapsed and DOC), 2 patients (20%) had not reached CR and died and 3 (33%) patients survived in CR. Median follow-up in HDC-treated patients was 12 months (range: 2–119 months). Results are summarised in Tables 2 and 3.
Survival with HLA-mismatched and HLA-matched grafts
Of 23 patients treated with HLA-matched grafts, 4 (17%) patients had succumbed due to treatment-related complications, 8 (35%) patients had relapsed and died, 5 (22%) patients had not reached CR and died and 7 (33%) patients had survived in CR. Median follow-up in patients treated with HLA-matched grafts was 12 months (range: 1–119 months) (Tables 2 and 3). Of 7 patients who received HLA-mismatched grafts, no one succumbed to treatment-related complications, 5 (71%) relapsed and died, 1 (14%) had not reached CR and died and 1 (14%) survived in CR. Median follow-up in patients treated with HLA-mismatched grafts was 5 months (range: 2–62 months) (Tables 2 and 3).
DLIs and GvHD
Three out of thirty patients received DLIs after allo-SCT (patients #4, #9, and #19; Table 2). Patient #4 was PR when she received two doses of 1 × 107 CD3-positive donor lymphocytes per kilogram body weight upfront without preparative chemo- or radiotherapy. She did not develop GvHD after DLI. Three weeks post DLI she showed tumour progression. Patient #9 relapsed after allo-SCT and received seven doses of donor lymphocytes in escalating doses (1 × , 3 × , 5 × , 10 × , 25 × , 50 × , and 100 × 106 CD3-positive cells per kg body weight) in combination with IL2 administration between DLI numbers 5 and 6 (at a total dose of 25 million units). Pretreatment before DLI consisted of surgical resection and chemotherapy (CWS 96 relapse protocol). The patient did not develop GvHD after DLI and was in CR for 97 months at the time of data censure. Patient #19 had relapsed PD after allo-SCT and received a single dose of 1 × 108 CD3-positive cells per kg body weight without preparative chemotherapy. Pretreatment consisted of radiotherapy of the relapse site. After DLI she did not develop GvHD but showed tumour progression. Altogether, despite high doses of donor lymphocytes none of these three patients developed GvHD after DLI.
Discussion
The rationale for treating cancer patients with allogeneic grafts is a hypothesised graft-vs-tumour effect of donor-derived cytotoxic T cells and/or natural killer cells that may unavoidably be given during infusion of haematopoietic stem cells for immune reconstitution or intentionally thereafter as DLI (Childs et al, 2000; Ueno et al, 2003; Bishop et al, 2004; Bregni et al, 2004; Kolb et al, 2004; Lundqvist and Childs, 2005; Mackensen et al, 2006; Rizzo et al, 2009; Reisner et al, 2011). Little is known about graft-vs-RMS effects in patients treated with allo-SCT and only few single-centre case experiences have been reported (Misawa et al, 2003; Donker et al, 2009; Ohta et al, 2011). In this study, we evaluated individual therapy outcomes of 30 patients with advanced RMS of all subtypes who became eligible for experimental allo-SCT. We focussed on toxicity, OS, PFS, and the possible presence of a graft-vs-RMS effect. As this is a retrospective study of a limited cohort with heterogeneous clinical courses, we did not carry out statistical significance calculations in regard to univariate group comparisons or multivariate analyses.
With a probability of 20%, 3-year OS in RMS patients treated with allo-SCT was comparable to the results of a recent meta-analysis reporting on the efficacy of HDC combined with autologous haematopoietic SCT in patients with advanced RMS (Peinemann et al, 2011). It has to be considered though, that survival data of four patients were censored within the 3 years following allo-SCT. In our analysis, with an overall DOC rate of 13%, toxicity seems to be controllable but yet not satisfactory. As death may be a competing event for toxicity onset, GvHD rates described here need to be interpreted with caution due to varying observation periods. An evaluation of possibly shared features of long-term survivors (here defined as CR for >2 years after allo-SCT) that could have led to cure remains elusive within our cohort. Similarly, a specific evaluation of the possible contribution of the donor’s immune system for RMS control is not feasible because patients had received multimodal therapies. Six patients were transplanted with residual disease. Of these patients, five patients were diagnosed with PD within 4 months after allo-SCT and one patient progressed 10 months after allo-SCT. All of these patients died of disease. However, it should be noted that a number of patients showed remarkable long PFS and/or OS after allo-SCT (Table 2). Four of five patients (#1, 2, 5, and 19) had chronic GvHD and survived for ⩾12 months after allo-SCT. Of these patients, patient #1 was transplanted without reaching CR and survived with stable disease for 10 months. The most impressive clinical course was seen in patient #9 (stage IV eRMS, disseminated and chemo-resistant disease after first-line treatment) who relapsed 28 months after transplantation, received seven times DLI thereupon, reached CR after surgery and chemotherapy with escalating DLI treatment and was surviving in CR for 97 months at the date of last follow-up. However, CR may have been due to surgery and chemotherapy rather than DLI. Again, it is not possible to precisely measure the role of infused T cells in this patient.
Several studies on the immunotherapeutical role of allo-SCT in patients with solid tumours and lympho-/myeloproliferative diseases could reveal or at least indicate the presence of a GvTE (Childs et al, 2000; Ueno et al, 2003; Bishop et al, 2004; Bregni et al, 2004; Kolb et al, 2004; Koscielniak et al, 2005; Lundqvist and Childs, 2005; Mackensen et al, 2006; Rizzo et al, 2009; Reisner et al, 2011). However, it remains unclear under which precise constellations this effect may become clinically relevant and if this effect is strong enough to outweigh the risk of severe GvHD. Recent progress in drug development for the control of severe GvHD has facilitated the flexibility on donor choice, that is, it has become possible to use grafts that were not fully HLA compatible (Reisner et al, 2011; Thiel et al, 2011b; Wernicke et al, 2011). Despite this, HLA-mismatched grafts remain associated with a higher risk of GvHD, but may yield higher graft-vs-tumour responses in a small spectrum of cancer entities (Reisner et al, 2011). The observation that a transplanted immune system may be able to control tumour progression or even cure patients, but on the other hand can cause life-threatening toxicity (Wernicke et al, 2011) has led to the development and the implementation of immunotherapeutical approaches using cancer/testis antigen selective cytotoxic T cells (Dalerba et al, 2001; Kuci et al, 2010) or NK cells (Lang et al, 2006; Perez-Martinez et al, 2009), either in an autologous (Morgan et al, 2006; Dudley et al, 2008) or in an allogeneic setting (Thiel et al, 2011a). Especially, the generation of T-cell receptor transgenic (Spranger et al, 2012) and/or chimaeric antigen receptor (CAR) (Marcus et al, 2011; Pegram et al, 2012) modified T cells against cancer/testis antigens appear to be a promising tool to facilitate specific anti-tumour responses.
The use of HDC regimens may elicit protective effects concerning disease relapse after autologous/allo-SCT in some paediatric sarcoma patients, but is bought with increased toxicity (Burdach et al, 2000). In contrast, RIC-based conditioning before allo-SCT for Ewing sarcomas was intended to facilitate a possible graft-vs-tumour effect, but was associated high relapse rates (Thiel et al, 2011b). The question which conditioning regimen is preferable has to be adressed in controlled prospective trials.
For patients with advanced paediatric sarcomas, it seems as if the different conventional conditioning approaches have reached a plateau considering rates of cure (Carli et al, 2004; Thiel et al, 2011b). Moreover, despite the presence of higher but improvingly controllable toxicity, it has to be questioned whether allo-SCT should be merely regarded upon as an experimental option to cure disease by itself. Allogeneic responses of donor T cells against non-self antigens may cause potent tissue rejection as seen in patients developing GvHD after allo-SCT, whereas autologous T cells may have developed central and peripheral tolerance to self-tissue including tumour tissue. Allogeneic T cells are not subjected to central tolerance and may overcome peripheral tolerance upon transfer if respective immunomodulatory pre- and post transplantation regimens are implemented. In this context, several immunomodulatory regimens for DLI, for example, lymphodepletion (Gattinoni et al, 2005), specific regulatory T cells depleting chemotherapy (Zhao et al, 2010), hyperthermia of tumour sites (Jolesch et al, 2012), blockade of immune checkpoint proteins (e.g., CTLA-4 and PD-1; Weber, 2010) and specific dendritic cell-based tumour vaccines (Ueno et al, 2010) have been proposed to enhance efficacy of immunotherapy. Furthermore, in sarcoma patients relapsing after allo-SCT an effect of increased chemosensitivity was recently reported, an observation that emphasises the need to explore the role of post-transplant chemotherapy regimens (Baird et al, 2012). The efficacy of each approach may be potentiated using individually tailored immunotherapeutic protocols combined with rescue chemotherapy and additional targeted therapy of crucial oncogenic pathways in tumour cells (Grunewald et al, 2012). Allo-SCT may therefore serve as a platform for additional immunotherapeutic approaches using, for example, (specific) DLI. It is still unclear how patients shall be conditioned to facilitate and/or enable curative immunotherapeutic effects. In our analysis, 3 out of 30 patients received high doses of DLI for relapse treatment after allo-SCT. Two of these patients received upfront high doses of DLI without prior dose escalation but did not develop GvHD afterwards. This observation hints at the presence of a possibly tumour mediated immune evasion (Mapara and Sykes, 2004).
With an OS probability of 20%, allo-SCT seems to be a feasible therapy option for patients with advanced RMS. Furthermore, the study population was heterogeneous in regard to patient and disease characteristics, previous treatments/outcomes of these treatments, reasons for allo-SCT, conditioning regimens, and observation periods. Therefore, the results have to be interpreted with caution. However, despite the limitations associated with all retrospective studies, we provide a systematic description of individual outcomes of a relatively large number of RMS patients treated with allo-SCT. Allo-SCT may constitute a suitable platform for immunotherapeutic approaches using, for example, (antigen-specific) DLI in the treatment of RMS patients with advanced disease in a multimodal setting comprising novel therapy approaches (Wan et al, 2006; Crose et al, 2012; Fulda, 2012). But the question under which circumstances it may be justified may only be answered in controlled clinical trials with prospective data collection.
Change history
12 November 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baird K, Fry TJ, Steinberg SM, Bishop MR, Fowler DH, Delbrook CP, Humphrey JL, Rager A, Richards K, Wayne AS, Mackall CL (2012) Reduced-intensity allogeneic stem cell transplantation in children and young adults with ultrahigh-risk pediatric sarcomas. Biol Blood Marrow Transplant 18 (5): 698–707.
Bishop MR, Fowler DH, Marchigiani D, Castro K, Kasten-Sportes C, Steinberg SM, Gea-Banacloche JC, Dean R, Chow CK, Carter C, Read EJ, Leitman S, Gress R (2004) Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol 22 (19): 3886–3892.
Bregni M, Bernardi M, Ciceri F, Peccatori J (2004) Allogeneic stem cell transplantation for the treatment of advanced solid tumors. Springer Semin Immunopathol 26 (1-2): 95–108.
Burdach S, van Kaick B, Laws HJ, Ahrens S, Haase R, Korholz D, Pape H, Dunst J, Kahn T, Willers R, Engel B, Dirksen U, Kramm C, Nurnberger W, Heyll A, Ladenstein R, Gadner H, Jurgens H, Go el U (2000) Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors. An update after long-term follow-up from two centers of the European Intergroup study EICESS. Stem-Cell Transplant Programs at Dusseldorf University Medical Center, Germany and St. Anna Kinderspital, Vienna, Austria. Ann Oncol 11 (11): 1451–1462.
Carli M, Colombatti R, Oberlin O, Bisogno G, Treuner J, Koscielniak E, Tridello G, Garaventa A, Pinkerton R, Stevens M (2004) European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: final results and analysis of prognostic factors. J Clin Oncol 22 (23): 4787–4794.
Carli M, Colombatti R, Oberlin O, Stevens M, Masiero L, Frascella E, Koscielniak E, Treuner J, Pinkerton CR (1999) High-dose melphalan with autologous stem-cell rescue in metastatic rhabdomyosarcoma. J Clin Oncol 17 (9): 2796–2803.
Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read EJ, Tisdale J, Dunbar C, Linehan WM, Young NS, Barrett AJ (2000) Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 343 (11): 750–758.
Chisholm JC, Marandet J, Rey A, Scopinaro M, de Toledo JS, Merks JH, O'Meara A, Stevens MC, Oberlin O (2011) Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol 29 (10): 1319–1325.
Crose LE, Etheridge KT, Chen C, Belyea B, Talbot LJ, Bentley RC, Linardic CM (2012) FGFR4 Blockade Exerts Distinct Antitumorigenic Effects in Human Embryonal versus Alveolar Rhabdomyosarcoma. Clin Cancer Res 18 (14): 3780–3790.
Dalerba P, Frascella E, Macino B, Mandruzzato S, Zambon A, Rosolen A, Carli M, Ninfo V, Zanovello P (2001) MAGE, BAGE and GAGE gene expression in human rhabdomyosarcomas. Int J Cancer 93 (1): 85–90.
Dantonello TM, Int-Veen C, Harms D, Leuschner I, Schmidt BF, Herbst M, Juergens H, Scheel-Walter HG, Bielack SS, Klingebiel T, Dickerhoff R, Kirsch S, Brecht I, Schmelzle R, Greulich M, Gadner H, Greiner J, Marky I, Treuner J, Koscielniak E (2009) Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol 27 (9): 1446–1455.
Donker AE, Hoogerbrugge PM, Mavinkurve-Groothuis AM, van de Kar NC, Boetes C, Hulsbergen-van de Kaa CA, Groot-Loonen JJ (2009) Metastatic rhabdomyosarcoma cured after chemotherapy and allogeneic SCT. Bone Marrow Transplant 43 (2): 179–180.
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26 (32): 5233–5239.
Fulda S (2012) Cell death pathways as therapeutic targets in rhabdomyosarcoma. Sarcoma 2012: 326210.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202 (7): 907–912.
Grunewald TG, Greulich N, Kontny U, Fruhwald M, Rutkowski S, Kordes U, Scheurlen W, Schmidt W, Stachel D, Metzler M, Mittler U, Graf N, Benesch M, Burdach S (2012) Targeted therapeutics in treatment of children and young adults with solid tumors: an expert survey and review of the literature. Klin Padiatr 224 (3): 124–131.
Jolesch A, Elmer K, Bendz H, Issels RD, Noessner E (2012) Hsp70, a messenger from hyperthermia for the immune system. Eur J Cell Biol 91 (1): 48–52.
Kolb HJ, Schmid C, Barrett AJ, Schendel DJ (2004) Graft-versus-leukemia reactions in allogeneic chimeras. Blood 103 (3): 767–776.
Koscielniak E, Gross-Wieltsch U, Treuner J, Winkler P, Klingebiel T, Lang P, Bader P, Niethammer D, Handgretinger R (2005) Graft-versus-Ewing sarcoma effect and long-term remission induced by haploidentical stem-cell transplantation in a patient with relapse of metastatic disease. J Clin Oncol 23 (1): 242–244.
Koscielniak E, Klingebiel TH, Peters C, Hermann J, Burdach ST, Bender-Gotze C, Muller-Weihrich ST, Treuner J (1997) Do patients with metastatic and recurrent rhabdomyosarcoma benefit from high-dose therapy with hematopoietic rescue? Report of the German/Austrian Pediatric Bone Marrow Transplantation Group. Bone Marrow Transplant 19 (3): 227–231.
Kuci S, Rettinger E, Voss B, Weber G, Stais M, Kreyenberg H, Willasch A, Kuci Z, Koscielniak E, Kloss S, von Laer D, Klingebiel T, Bader P (2010) Efficient lysis of rhabdomyosarcoma cells by cytokine-induced killer cells: implications for adoptive immunotherapy after allogeneic stem cell transplantation. Haematologica 95 (9): 1579–1586.
Lang P, Pfeiffer M, Muller I, Schumm M, Ebinger M, Koscielniak E, Feuchtinger T, Foll J, Martin D, Handgretinger R (2006) Haploidentical stem cell transplantation in patients with pediatric solid tumors: preliminary results of a pilot study and analysis of graft versus tumor effects. Klin Padiatr 218 (6): 321–326.
Lundqvist A, Childs R (2005) Allogeneic hematopoietic cell transplantation as immunotherapy for solid tumors: current status and future directions. J Immunother 28 (4): 281–288.
Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R (2006) Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 24 (31): 5060–5069.
Mapara MY, Sykes M (2004) Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol 22 (6): 1136–1151.
Marcus A, Waks T, Eshhar Z (2011) Redirected tumor-specific allogeneic T cells for universal treatment of cancer. Blood 118 (4): 975–983.
Misawa A, Hosoi H, Tsuchiya K, Iehara T, Sawada T, Sugimoto T (2003) Regression of refractory rhabdomyosarcoma after allogeneic stem-cell transplantation. Pediatr Hematol Oncol 20 (2): 151–155.
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314 (5796): 126–129.
Ohta H, Hashii Y, Yoshida H, Kusuki S, Tokimasa S, Yoneda A, Fukuzawa M, Inoue N, Hara J, Kusafuka T, Ozono K (2011) Allogeneic hematopoietic stem cell transplantation against recurrent rhabdomyosarcoma. J Pediatr Hematol Oncol 33 (1): e35–e38.
Pappo AS, Shapiro DN, Crist WM, Maurer HM (1995) Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol 13 (8): 2123–2139.
Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ (2012) Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119 (18): 4133–4141.
Peinemann F, Kroger N, Bartel C, Grouven U, Pittler M, Erttmann R, Kulig M (2011) High-dose chemotherapy followed by autologous stem cell transplantation for metastatic rhabdomyosarcoma—a systematic review. PLoS One 6 (2): e17127.
Perez EA, Kassira N, Cheung MC, Koniaris LG, Neville HL, Sola JE (2011) Rhabdomyosarcoma in children: a SEER population based study. J Surg Res 170 (2): e243–e251.
Perez-Martinez A, Leung W, Munoz E, Iyengar R, Ramirez M, Vicario JL, Lassaletta A, Sevilla J, Gonzalez-Vicent M, Madero L, Diaz-Perez MA (2009) KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer 53 (1): 120–124.
Reisner Y, Hagin D, Martelli MF (2011) Haploidentical hematopoietic transplantation: current status and future perspectives. Blood 118 (23): 6006–6017.
Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers ME, Friedman DL, Horowitz MM, Wingard JR, Deeg HJ (2009) Solid cancers after allogeneic hematopoietic cell transplantation. Blood 113 (5): 1175–1183.
Scrucca L, Santucci A, Aversa F (2007) Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 40 (4): 381–387.
Spranger S, Jeremias I, Wilde S, Leisegang M, Starck L, Mosetter B, Uckert W, Heemskerk MH, Schendel DJ, Frankenberger B (2012) TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood 119 (15): 3440–3449.
Stevens MC (2005) Treatment for childhood rhabdomyosarcoma: the cost of cure. Lancet Oncol 6 (2): 77–84.
Stevens MC, Rey A, Bouvet N, Ellershaw C, Flamant F, Habrand JL, Marsden HB, Martelli H, Sanchez de Toledo J, Spicer RD, Spooner D, Terrier-Lacombe MJ, van Unnik A, Oberlin O (2005) Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology—SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol 23 (12): 2618–2628.
Thiel U, Pirson S, Muller-Spahn C, Conrad H, Busch DH, Bernhard H, Burdach S, Richter GH (2011a) Specific recognition and inhibition of Ewing tumour growth by antigen-specific allo-restricted cytotoxic T cells. Br J Cancer 104 (6): 948–956.
Thiel U, Wawer A, Wolf P, Badoglio M, Santucci A, Klingebiel T, Basu O, Borkhardt A, Laws HJ, Kodera Y, Yoshimi A, Peters C, Ladenstein R, Pession A, Prete A, Urban EC, Schwinger W, Bordigoni P, Salmon A, Diaz MA, Afanasyev B, Lisukov I, Morozova E, Toren A, Bielorai B, Korsakas J, Fagioli F, Caselli D, Ehninger G, Gruhn B, Dirksen U, Abdel-Rahman F, Aglietta M, Mastrodicasa E, Torrent M, Corradini P, Demeocq F, Dini G, Dreger P, Eyrich M, Gozdzik J, Guilhot F, Holler E, Koscielniak E, Messina C, Nachbaur D, Sabbatini R, Oldani E, Ottinger H, Ozsahin H, Schots R, Siena S, Stein J, Sufliarska S, Unal A, Ussowicz M, Schneider P, Woessmann W, Jurgens H, Bregni M, Burdach S (2011b) No improvement of survival with reduced- versus high-intensity conditioning for allogeneic stem cell transplants in Ewing tumor patients. Ann Oncol 22 (7): 1614–1621.
Tomblyn M, Lazarus HM (2008) Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant 42 (9): 569–579.
Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, Oh S, Fay J, Pascual V, Banchereau J, Palucka K (2010) Harnessing human dendritic cell subsets for medicine. Immunol Rev 234 (1): 199–212.
Ueno NT, Cheng YC, Rondon G, Tannir NM, Gajewski JL, Couriel DR, Hosing C, de Lima MJ, Anderlini P, Khouri IF, Booser DJ, Hortobagyi GN, Pagliaro LC, Jonasch E, Giralt SA, Champlin RE (2003) Rapid induction of complete donor chimerism by the use of a reduced-intensity conditioning regimen composed of fludarabine and melphalan in allogeneic stem cell transplantation for metastatic solid tumors. Blood 102 (10): 3829–3836.
Wan X, Shen N, Mendoza A, Khanna C, Helman LJ (2006) CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia 8 (5): 394–401.
Weber J (2010) Immune checkpoint proteins: a new therapeutic paradigm for cancer—preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37 (5): 430–439.
Wernicke CM, Grunewald TG, Juenger H, Kuci S, Kuci Z, Koehl U, Mueller I, Doering M, Peters C, Lawitschka A, Kolb HJ, Bader P, Burdach S, von Luettichau I (2011) Mesenchymal stromal cells for treatment of steroid-refractory GvHD: a review of the literature and two pediatric cases. Int Arch Med 4 (1): 27.
Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B (2010) Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res 70 (12): 4850–4858.
Acknowledgements
This work was supported by by grants to SB from the Wilhelm Sander-Stiftung (2006.109.1), Else Kröner–Fresenius–Stiftung (SB and GR; P31/08//A123/07), BMBF (SB and GR; TranSarNet FK:01GM0870), the Deutsche Forschungsgemeinschaft (DFG, GR3728/2-1 to TG), AmGen and Chugai and the Deutsche Kinderkrebsstiftung (SB and GR; DKS 2010.07) and to SB, GR, and UT from the BMBF (TranSarNet 01GM1104B). We wish to thank all patients and their families as well as all data managers, physicians, and nurses for their contribution to this study. Petra Wolf is especially acknowledged for helpful advice in the statistical evaluation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Thiel, U., Koscielniak, E., Blaeschke, F. et al. Allogeneic stem cell transplantation for patients with advanced rhabdomyosarcoma: a retrospective assessment. Br J Cancer 109, 2523–2532 (2013). https://doi.org/10.1038/bjc.2013.630
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.630
Keywords
This article is cited by
-
The Role of Natural Killer Cells as a Platform for Immunotherapy in Pediatric Cancers
Current Oncology Reports (2019)
-
Haploidentical allogeneic hematopoietic stem cell transplantation in patients with high-risk soft tissue sarcomas: results of a single-center prospective trial
Bone Marrow Transplantation (2018)
-
Diagnosis and Management of Rhabdomyosarcoma in Children and Adolescents: ICMR Consensus Document
The Indian Journal of Pediatrics (2017)
-
Favorable NK cell activity after haploidentical hematopoietic stem cell transplantation in stage IV relapsed Ewing’s sarcoma patients
Bone Marrow Transplantation (2015)
-
Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015
Bone Marrow Transplantation (2015)