Abstract
Background:
Vascular endothelial growth factor (VEGF)-mediated angiogenesis mediates tumour growth and metastasis. Meningiomas are primarily benign, slow-growing, highly vascularised tumours. Aside from VEGF, there is little data on the function of major angiogenic proteins in meningiomas.
Methods:
The VEGFA, platelet-derived growth factor B (PDGFB), and their respective receptors – VEGF receptor 2 (KDR) and PDGF receptor β (PDGFRβ) – were quantified using real-time PCR and a TaqMan Protein Assay in meningiomas in vivo and in vitro. The effect of VEGFA and PDGFB on cell proliferation and the tyrosine phosphorylation of PDGFRβ were examined.
Results:
Most meningiomas displayed no KDR protein expression but elevated PDGFRβ levels. Exogenous VEGFA stimulation significantly increased cell proliferation. The PDGFRβ inhibition before stimulation with VEGFA abolished the proliferative stimuli. The VEGFA induced concentration-dependent PDGFRβ tyrosine phosphorylation comparable to PDGFB-induced PDGFRβ tyrosine phosphorylation. The PDGFRβ inhibitors gambogic acid, sunitinib, and tandutinib equally impaired the migration of meningioma cells. In addition, gambogic acid suppressed the VEGFA-induced PDGFRβ tyrosine phosphorylation.
Conclusion:
Collectively, our data suggest that VEGFA primarily regulates VEGF-mediated migration through PDGFRβ in meningiomas. The inhibitory effect of gambogic acid and tandutinib against meningioma growth in vitro suggests that selective PDGFRβ inhibitors, in combination with VEGF inhibitors, should be evaluated further as potential therapies for recurrent and malignant meningiomas.
Similar content being viewed by others
Main
Meningiomas are the most common type of intracranial tumours. They originate from the arachnoidal cap cells of the meningeal cover of the spinal cord and brain and account for ∼13–26% of all intracranial neoplasms (Riemenschneider et al, 2006; Louis et al, 2007). The conventional treatment for meningiomas is surgery (McMullen and Stieber, 2004). The 5-year recurrence rates after complete resection in benign, atypical, and anaplastic meningiomas were 3%, 38%, and 78%, respectively, for World Health Organisation (WHO) grade I meningiomas, the recurrence rate increased after incomplete resection from 7.5% to 9.8% at 10 years and 9.3% to 25.6% at 20 years (Jääskeläinen, 1986; Nakasu et al, 2009). The potential for malignancy is unresolved, due to a frequent discordance between histology and biology (Mahmood et al, 1993; Schittenhelm et al, 2006). Meningiomas are categorised into three grades by the WHO, and there are several subtypes based on histology.
Angiogenesis is essential for the enlargement of any solid tumour. Vascular endothelial growth factor (VEGF) is the key regulator of pathological angiogenesis (Ferrara et al, 2003). The VEGFA is the most abundant member of the VEGF family (Holmes and Zachary, 2005; Yamazaki and Morita, 2006), which comprising several isoforms, such as VEGF165, through alternative splicing. There are three VEGF receptor tyrosine kinases (VEGFR1–3), but VEGFA only binds to VEGFR1 (FLT1) and VEGFR2 (KDR), and most of the signal transduction is mediated by the latter (Cross et al, 2003).
The VEGF and VEGFRs mediate angiogenesis in various brain tumours (Nishikawa et al, 1998; Huang et al, 2005). Although VEGF is upregulated in meningiomas (Provias and Claffey, 1997; Christov et al, 1999; Lamszuz et al, 2000; Park et al, 2000; Yamasaki, 2000), it is unknown whether the degree of malignancy correlates with the expression of VEGF (Provias and Claffey, 1997; Lamszuz et al, 2000; Yamasaki, 2000).
Platelet-derived growth factor B (PDGFB) is another critical factor that promotes the recruitment and proliferation of vascular cells (Yancopoulos et al, 2000). The PDGF signalling indirectly regulates angiogenesis. Platelet-derived growth factor receptor β (PDGFRβ) can induce the transcription and secretion of VEGF. The PDGFB and its receptors (PDGFR) are expressed in several human cancers, such as gliomas, breast cancer, colorectal cancer, and myelomonocytic leukaemia (Yu et al, 2003). The PDGFRβ and PDGFB are expressed in meningiomas, and their overexpression correlates with the WHO grade (Wang et al, 1990; Yang, 2001).
Several therapeutic agents that specifically target the angiogenic pathway, such as sunitinib and tandutinib, have entered clinical development. Both compounds are tyrosine kinase inhibitors, but while sunitinib inhibits at least eight receptor protein tyrosine kinases, including KDR and PDGFRβ (Mendel et al, 2003), tandutinib only acts on PDGFRβ, Fms-like tyrosine kinase 3 (FLT3), and c-Kit (Kelly et al, 2002). Sunitinib is used to treat metastatic renal cell cancer and gastrointestinal stromal tumours (Motzer et al, 2006), and tandutinib is in early-phase clinical evaluation for acute myeloid leukaemia (Cheng and Paz, 2008).
Recently, Liu et al (2010) demonstrated that gambogic acid inhibits cell migration by suppressing PDGFRβ tyrosine phosphorylation. Gambogic acid is the major active compound in gamboge, a resin from Garcinia hanburyi. Gambogic acid has potent apoptotic activity against several cancer cell lines in vitro and in vivo, including glioblastoma cell lines (Gu et al, 2008; Qiang et al, 2008).
In this study, we analysed the expression of VEGFA, KDR, PDGFB, and PDGFRβ in vitro and in vivo using quantitative real-time PCR (qRT–PCR) and the TaqMan Protein Assay. We examined the biological significance of VEGFA signalling through PDGFRβ by comparing the effects of VEGFA and PDGFB on meningioma cell proliferation and PDGFRβ tyrosine phosphorylation. We also determined the effects of gambogic acid and two tyrosine kinase inhibitors of PDGFRβ (sunitinib and tandutinib) on meningiomas in vitro.
Materials and methods
Tumour specimens and cell culture
The meningioma surgical specimens, arachnoidea, and dura were obtained from the Neurosurgical Department of the University of Tuebingen according to the guidelines of the ethics committee. The primary cultures were established from tumour tissue samples within 30 min of surgical removal. Samples were washed in phosphate-buffered saline (PBS), reduced, passed through a filter, and placed in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 0.1% 10 mg ml−1 gentamycin (Invitrogen, Grand Island, NY, USA). The cells were plated in 25-mm2 tissue culture flasks and incubated at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 3–4 days, and the cultures were split with 600 μl Accutase (PAA, Pasching, Austria). The viable cells were stored in liquid nitrogen in 90% medium/10% dimethyl sulphoxide (DMSO). To verify the presence of tumour cells, the primary meningioma cultures were tested immunocytochemically for the expression of epithelial membrane antigen and vimentin. Both proteins are expressed in most meningiomas. The cells were also tested for the expression of CD34, an endothelial cell marker. The malignant meningioma IOMM-Lee cell line was a kind gift from A Lal (Department of Neurological Surgery, University of California, San Francisco, CA, USA). The IOMM-Lee cell line was tested using cytogenic characterisation. The human umbilical vein endothelial cells (HUVECs) (Genlantis+Cell Applications, Inc., San Diego, CA, USA) were cultured in endothelial cell growth medium (Genlantis, San Diego, CA, USA). The human prenatal neural cells (HNCs; dv Biologics, Costa Mesa, CA, USA) were cultured in Neurobasal A medium supplemented with B27 and 10 ng ml−1 FGF basic (Invitrogen). The human adult mesothelial cells (HMCs; Zen-Bio, Research Triangle Park, NC, USA) were cultured in mesothelial cell growth medium (MSO-1; Zen-Bio).
TaqMan protein assays
The following biotinylated antibodies (R&D Systems, Minneapolis, MN, USA) were used: VEGFA (BAF293), KDR (BAF357), PDGFB (BAF220), and PDGFRβ (BAF385). Phospho-PDGFRβ (AF1767) and phospho-KSR (AF1766) were labelled with biotin using the Biotin-XX Microscale Protein Labelling Kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. The biotinylated antibodies were labelled with the TaqMan Protein Assay Open Kit (Applied Biosystems, Foster City, CA, USA) as per manufacturer’s instructions. The specificity of each assay was determined using the recombinant proteins VEGFA, PDGFB (Invitrogen Inc.), KDR, and PDGFRβ (R&D Systems). Applied Biosystems recommends ΔCt values for recombinant proteins greater than 5. All ΔCt values for the above-mentioned assays were greater than 5 (VEGFA ΔCt=8.32; KDR ΔCt=7.13; PDGFB ΔCt=8.44; and PDGFRβ ΔCt=5.17). The assays for phospho-PDGFRβ and phospho-KDR consisted of probe A PDGFRβ and probe B phospho-PDGFRβ and probe A KDR and probe B phospho-KDR, respectively. The specificity for phosphorylated PDGFRβ and KDR was determined using the recombinant proteins for PDGFRβ and KDR, which the respective assay could not detect.
The total meningioma protein in vitro was isolated directly from primary cell cultures before being split using the PARIS kit (Ambion, Inc., Austin, TX, USA). In vivo meningioma and dura proteins were isolated from fresh tissue using the Protein Quant Sample Lysis kit (Applied Biosystems) according to manufacturer’s protocol. A two-fold serial dilution was prepared according to manufacturer’s protocol with a cell lysate input per well of 5 μg for meningiomas and dura. Total human brain protein (Biochain, Hayward, CA, USA) was used as a reference with an input amount of 25 μg. A TaqMan Protein Assay was performed using the TaqMan Protein Assay Core Reagents Kit with Master Mix (Applied Biosystems) according to manufacturer’s instructions. qRT–PCR was performed on the StepOne Real-Time PCR System (Applied Biosystems) using the standard instrument protocol for the TaqMan Protein Assay. The data were analysed using the ProteinAssist Software 1.0 (Applied Biosystems), which uses the ΔCt2 method to calculate relative protein expression between untreated controls and treated samples. The sample dilutions were assayed, and the resulting Ct values were normalised to the sample input, which requires accurate protein quantitation. Every 48-well plate included a no-protein control (NPC) to calculate the ΔCt values (Ct value (sample)−Ct value (NPC)). Then, a linear range was generated for each sample, and a ΔCt threshold was designated. The fold change between samples was calculated between the crossover points of each linear trend line at the ΔCt threshold.
RNA isolation and reverse transcription
Total meningioma RNA was isolated in vitro directly from primary cell cultures before being split. Meningioma, arachnoidea, and dura RNA were isolated in vivo from fresh tissue using the PARIS kit (Ambion, Inc.) according to manufacturer’s protocol. The RNA was treated with DNAfree (Ambion, Inc.) to remove residual genomic DNA. The RNA concentration and 260/280 absorbance ratios were measured in triplicate on an Eppendorf Biophotometer (Eppendorf, Hamburg, Germany). The integrity of the RNA samples was confirmed by electrophoresis on a 2% SYBR Green agarose gel (Invitrogen Inc.). The criteria for the inclusion of RNA samples were a 260/280 ratio of ∼2 (1.9–2.2) and a 28S/18S ratio of ⩾1.7. The probes were stored at −80°C until use.
For normal brain samples, FirstChoice Human Brain Reference RNA (Ambion, Inc.), which pools RNA from various donors and several regions of the brain, was used. The RNA samples were treated with DNAfree (Ambion Inc.). The total RNA (1 μg) was reverse transcribed to cDNA using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems) in 20 μl according to manufacturer’s protocol.
Quantitative real-time PCR
TaqMan qRT–PCR was performed in triplicate in 48-well reaction plates with StepOne (Applied Biosystems). Each reaction contained 1 μl of cDNA (5 ng μl−1) in 20 μl of reaction mix, containing 10 μl of TaqMan Gene Expression Master Mix (Applied Biosystems) and 1 μl of TaqMan Gene Expression Assay (Applied Biosystems). The following TaqMan Gene Expression primers were used: VEGFA (Hs99999070_m1), KDR (Hs00911700_m1), PDGFB (Hs00234042_m1), and PDGFRB (Hs00387364_m1). All primers span an exon junction and will not detect genomic DNA. The cycling conditions were as follows: an initial hold at 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min for 40 cycles.
Reverse transcriptase-negative controls and no-template controls (without cDNA) were included. RPL37A (Hs01102345_m1), which is the optimal reference for meningiomas and control tissues (Pfister et al, 2011), was used as the reference gene for relative quantification. The data were collected and analysed quantitatively using StepOne, v2.2 (Applied Biosystems). The relative gene expression data were analysed using the 2−ΔΔCt method (Erickson et al, 2007).
Immunohistochemistry
Four-micron sections that contained human meningiomas were cut from formalin-fixed paraffin-embedded tissue blocks. The slides were deparaffinised in a series of histolene (Engelbrecht, Edermuende, Germany) and alcohol solutions. The Vectastain Elite Universal Kit (Vector Laboratories, Burlingame, CA, USA) was used according to manufacturer’s protocol. Briefly, the slides were treated with an antigen unmasking solution (citrate buffer, pH 6.0; 94°C; 30 min), rinsed with PBS, and bathed in 3% H2O2 for 10 min, followed by blocking with avidin/biotin (Vector Laboratories).
The slides were incubated overnight at 4°C with a primary VEGFA rabbit polyclonal antibody (sc-152; Santa Cruz, Santa Cruz, CA, USA) at 1 : 60, a KDR rabbit polyclonal antibody (Ab2349; Abcam, Cambridge, MA, USA) at 1 : 100, a PDGFB rabbit polyclonal antibody (sc-127; Santa Cruz) at 1 : 100, or a PDGFRβ rabbit polyclonal antibody (sc-339; Santa Cruz) at 1 : 200. Next, the slides were washed three times with PBS, a biotinylated secondary antibody was applied, and a premixed avidin-biotin-peroxide complex was added (ABC; Vector Laboratories). The stains were developed with Vector Novared (Vector Laboratories). The positive controls were selected for each specimen as follows: VEGFA+KDR (human kidney), PDGFRβ (human placenta), and PDGFB (human pancreas). The negative control slides were incubated either without primary antibody or with a blocking peptide. The sections were counterstained with haematoxylin and examined using light microscopy. The immunohistochemical samples were graded from 0% to 100% based on the percentage of cells that were positive relative to the total tissue.
Growth factors, inhibitors, and drugs
VEGF165 (VEGFA, PHC9391; Invitrogen Inc.) was reconstituted in sterile distilled water, aliquoted, and stored at −20°C until use. PDGF-BB (PDGFB, PHG0041; Invitrogen Inc.) was reconstituted in 100 mM acetic acid and 0.1% BSA, aliquoted, and stored at −20°C until use. The VEGFR2 (KDR) kinase inhibitor VI (Ki8751) (676484; Merck, Darmstadt, Germany) inhibits KDR with an IC50 of 0.9 nM and has no effect on PDGFRβ at concentrations as high as 10 μ M. PDGFR tyrosine kinase inhibitor III (521232; Merck) inhibits PDGFRβ with an IC50 of 80 nM with no inhibitory effect on KDR. Both inhibitors were dissolved in DMSO (Sigma-Aldrich, St Louis, MO, USA) and stored at −20°C. Sunitinib malate (SU-11248, Sutent) and gambogic acid were obtained from Santa Cruz, and tandutinib (MLN518) was purchased from Selleck Chemicals (Houston, TX, USA). The stock solutions of sunitinib, tandutinib, and gambogic acid were dissolved in DMSO, stored at −20°C, and diluted in fresh medium immediately before use.
Proliferation assay
The IOMM-Lee cells and primary meningioma cells in mid-log phase were seeded onto 24-well culture plates in DMEM containing 10% FBS for 24 h. Subsequently, the cells were treated with increasing concentrations of VEGFA (0, 5, 10, 20, 50, and 100 ng ml−1) in serum-free medium for 4 h. After 3 h of treatment, 5-Bromo-2′-deoxy-uridine (BrdU) was added to each well (1 : 1000). After incubation, BrdU was detected using the BrdU Labelling and Detection Kit II (Roche, Mannheim, Germany) according to manufacturer’s protocol. The cell number was evaluated under a light microscope.
GAP assay
Primary meningioma cells (0.3 × 106 cells per ml) were seeded onto 24-well tissue culture dishes using the Radius 24-Well Cell Migration Assay (Cell Biolabs, Inc., San Diego, CA, USA) according to manufacturer’s instructions. After 24 h of incubation to allow spreading, the gel was removed. The compounds were added in a medium that contained 10% FBS, and the cells were incubated for 24 h with various concentrations of sunitinib, tandutinib, and gambogic acid (0.125–10 μg ml−1) to determine their effect on cell migration. The meningioma cell migration was observed and photographed under an inverted microscope before and after 24 h of inhibition with sunitinib, tandutinib, or gambogic acid. The cells were analysed using CellProfiler (www.cellprofiler.org) (Carpenter et al, 2006).
Statistical analysis
Statistical analysis was performed with GraphPad Prism, V5.03 (GraphPad Software, La Jolla, CA, USA). All results are expressed as mean values±standard deviation (s.d.). Statistical comparisons between two groups were made using the Mann–Whitney test. For statistical comparisons between a group and the human brain reference, the Wilcoxon signed-rank test was used. Significant differences between sets of data were determined using a paired t-test. The level of significance was set at P<0.05. All tests were two-sided.
Results
Elevated PDGFRβ expression in meningiomas
The RNA and protein expression was measured in meningiomas in vitro and in vivo (Table 1). The RNA expression was quantified using qRT–PCR with RPL37A for normalisation. The protein levels were measured with a TaqMan Protein Assay. The human brain was used as a reference. Only three samples of arachnoidea, the cap cells from which meningiomas originate, were obtained. Due to the small sample size and the structure of the arachnoidea, only a small amount of RNA and protein was isolated. The RNA and protein levels of several angiogenesis genes in the arachnoidea and dura were similar, with the exception of KDR, which was significantly higher in arachnoidea (P=0.002). Thus, we chose to measure the gene expression in the dura, from which ample RNA and protein could be isolated. Due to the small number of arachnoidea samples, the expression of angiogenesis genes in meningiomas was compared with the expression in dura to detect significant changes in expression levels, except for KDR. All results are expressed as the relative gene expression levels (RQ) to brain (RQBrain=1) for RNA or as the fold change with brain as reference (fold changeBrain=1) for protein.
The PDGFRβ RNA and protein expression was significantly higher in meningiomas compared with the brain (P<0.0001) (Figure 1A). The PDGFRβ RNA expression in vitro was significantly decreased in WHO °III (PWHO °I vs WHO °III=0.0198 and PWHO °II vs WHO °III=0.0004), but there was no difference between WHO °I and °II. The in vivo RNA expression of PDGFRβ was significantly decreased in meningiomas compared with dura (P=0.0014) but displayed no significant changes between WHO grades. In contrast to the in vitro RNA levels, the in vitro protein expression of PDGFRβ was significantly decreased in dura compared with meningioma (P=0.042). The PDGFRβ RNA and protein levels in vitro were significantly higher than those detected in vivo (PRNA=0.0002; PProtein=0.0045).
The in vitro and in vivo RNA and protein expression of angiogenetic proteins in meningiomas and dura. Expression of RNA was quantified using qRT–PCR with RPL37A for normalisation. The protein levels were measured using the TaqMan Protein Assay. The data for RNA are expressed as the relative gene expression level (RQ) compared with the brain (RQBrain=1). The data for protein are expressed as fold change, with the brain as the reference. (A) The PDGFRβ RNA and protein expression was significantly higher in meningiomas compared with the brain. The in vitro PDGFRβ RNA expression was significantly decreased in WHO °III, *PRNA WHO °I vs WHO °III=0.0198; **PRNA WHO °II vs WHO °III=0.004; the PDGFRβ RNA and protein levels in vitro were significantly higher compared with those in vivo. #PRNA in vitro vs in vivo=0.0002; ##PProtein in vitro vs in vivo=0.045. The bars represent the mean±s.d. (B) The KDR RNA and protein expression levels were significantly elevated in arachnoidea compared with dura, *PRNA=0.002; **PProtein=0.0167. In meningiomas, the KDR RNA and protein expression levels were significantly decreased, #PRNA=0.001; ##PProtein=0.0014. (C) Most meningiomas displayed no KDR protein expression. In addition, the KDR RNA expression was extremely low in most meningiomas (medianin vitro=0.17; medianin vivo=0.28). The symbols represent single data points. The horizontal bars represent the median. (D) The VEGFA RNA was significantly downregulated in vivo and in vitro in meningiomas compared with arachnoidea (*P=0.0331) and dura (**P=0.0023). In contrast, the VEGFA protein expression in vitro was significantly higher in meningiomas compared with dura (***P=0.034). (E) The PDGFB RNA and protein levels were significantly downregulated in meningiomas compared with dura, *PRNA=0.0076; **PProtein=0.0082.
Most meningiomas displayed no KDR protein expression
The KDR RNA and protein expression was significantly elevated in arachnoidea compared with dura (PRNA=0.002; PProtein=0.0167) (Figure 1B). In addition, the KDR levels were higher in dura compared with the brain (PRNA=0.002; PProtein=0.0156). In contrast, the KDR RNA and protein expression in meningiomas was significantly decreased (PRNA=0.001; PProtein=0.0014). In fact, KDR could not be detected in most meningiomas. Only 4 of 38 (10.3%) primary meningioma cell cultures displayed positive KDR protein expression (Figure 1C). In all, 8 of the 37 (21.6%) meningiomas that were isolated directly from fresh surgical specimens were KDR positive. The KDR RNA expression was extremely low in most meningiomas (medianin vitro=0.17; medianin vivo=0.28). Only five in vitro (6.4%) and nine in vivo (17.3%) meningiomas displayed an RQ of >0.5. The KDR expression was two-fold higher in meningiomas in vivo, but a significant difference was only displayed between the in vivo and in vitro RNA levels (P=0.0043). There were no significant differences in the expression levels between WHO grades. For comparison, the RNA and protein expression levels of PDGFRβ and KDR in eight cancer cell lines were determined (data not shown). The PDGFRβ expression was on average 97% lower in cancer cell lines compared with brain expression, and KDR expression was on average 15% higher.
Broad distribution of VEGFA expression and low PDGFB expression in meningiomas
VEGFA RNA in vivo and in vitro was significantly downregulated in meningiomas compared with arachnoidea and dura (arachnoidea: Pin vitro=0.0346; Pin vivo=0.0331; dura: Pin vitro=0.012; Pin vivo=0.0023) (Figure 1D). In contrast, the in vitro VEGFA protein expression was significantly higher in meningiomas compared with dura (P=0.034). In addition, the VEGFA expression in meningiomas displayed a broad distribution, especially in vivo. For the in vivo RNA expression, the minimum was 0.02 and the maximum was 132.5 with a median of 0.725. In vitro, the lowest VEGFA expression was 0.22 and the highest expression was 38.34 with a median of 1.42. For the in vivo protein expression, the minimum was 0.001 and the maximum was 80 with a median of 2.6. The lowest VEGFA expression in vitro was 0.029 and the highest expression was 65 with a median of 0.92.
The PDGFB RNA and protein levels were significantly downregulated in meningiomas compared with dura (RNA: Pin vitro=0.0002; Pin vivo=0.0076; protein: Pin vitro=0.003; Pin vivo=0.0082), as shown in Figure 1E. There were no significant differences in expression levels between WHO grades.
Immunohistochemical staining of angiogenetic proteins
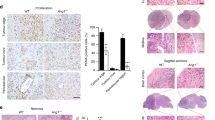
Immunohistochemistry was performed using 54 surgical human meningioma specimens that included – 28 WHO °I, 21 WHO °II, and 5 WHO °III meningiomas. Figure 2A shows a representation of the staining in a negative control (A), a positive control (kidney) for VEGFA (B), positive VEGFA expression in a meningioma (C), a positive control (kidney) for KDR (D), positive KDR expression in a meningioma (E), negative KDR expression in a meningioma (F), a positive control (placenta) for PDGFRβ (G), and positive PDGFRβ expression in two meningiomas (H+I). All angiogenetic proteins displayed cytoplasmic and membranous staining in neoplastic cells. Expression of KDR and PDGFRβ was also found in blood vessels. Figure 2B–E shows the expression of angiogenetic proteins based on the percentage of cells that were positive relative to the total tissue sample.
Immunohistochemical staining of angiogenic proteins in meningiomas. The paraffin-embedded, formalin-fixed sections of meningiomas were stained with antibodies against VEGFA, KDR, PDGFB, and PDGFRβ. Positive immunoreactivity appears as red staining (Vector NovaRed). The stains were graded from 0% to 100% based on the percentage of cells that were positive relative to the total tissue. (A) The panels are a representation of staining in a negative control (a), a positive VEGFA control (kidney) (b), positive VEGFA expression in a meningioma (c), a positive KDR control (kidney) (d), positive KDR expression in a meningioma (e), negative KDR expression in a meningioma (f), a positive PDGFRβ control (placenta) (g), and positive PDGFRβ expression in two meningiomas (h+i). (B) All 54 meningiomas (28 WHO °I, 21 WHO °II, and 5 WHO °III) stained positive for VEGFA and PDGFRβ. Forty-seven meningiomas (87%) were positive for KDR with a median percentage of 2.5%. (C) The PDGFRβ expression differed between grades (PWHO °I vs WHO °II=0.0426; PWHO °II vs WHO °III=0.0315). (D) The VEGFA expression did not differ between WHO °I and WHO °II. In contrast, the VEGFA expression was higher in grade WHO °III vs grade WHO °II meningiomas (P=0.02). (E) The KDR expression displayed no significant differences between WHO grades. The box plots represent maximum and minimum values with the median.
All meningiomas stained positive for VEGFA and PDGFRβ. The median percentage of PDGFRβ-positive cells in meningiomas was 20% (range 2–80%). As shown in Figure 2C, PDGFRβ expression differed between grades (PWHO °I vs WHO °II=0.0426; PWHO °II vs WHO °III=0.0315). The median percentage of VEGFA-positive cells was 30% (range 2–90%). The expression in meningiomas did not differ between WHO °I and WHO °II. In contrast, VEGFA expression was higher in grade WHO °III vs grade WHO °II meningiomas (P=0.02) (Figure 2D).
Forty-seven meningiomas (87%) were positive for KDR with a median percentage of 2.5%. Most meningiomas (67%) showed low KDR expression with up to 5% positive cells. Eleven meningiomas (20%) demonstrated 10–30% positive staining. Expression of KDR was not significantly different between the WHO grades (Figure 2E).
Fifty-three meningiomas (98%) were positive for PDGFB (median percentage 20% and maximum 90%). There were no differences in PDGFB expression between the WHO grades.
Proliferative response to exogenous VEGFA in meningiomas
To investigate the biological significance of the elevated PDGFRβ expression and the mostly non-expression of KDR in meningiomas IOMM-Lee cells, a malignant proliferating meningioma cell line, were cultured with increasing amounts (0, 5, 10, 20, 50, and 100 ng ml−1) of VEGFA and PDGFB for 4 h in serum-free medium. As shown in Figure 3A, cell proliferation was significantly increased after treatment with 10 ng ml−1 VEGFA (+42% compared with control conditions, P<0.05), 20 ng ml−1 VEGFA (+87.4% compared with control conditions, P<0.005), 50 ng ml−1 VEGFA (+94.6% compared with control conditions, P<0.005), and 100 ng ml−1 VEGFA (+99% compared with control conditions, P<0.001). The control cells were also incubated with PDGFB. As shown in Figure 3B, cell proliferation was similarly increased after treatment with 10 ng ml−1 PDGFB (+46.2% compared with control conditions, P<0.05), 20 ng ml−1 PDGFB (+71.2% compared with control conditions, P<0.005), and 50 ng ml−1 PDGFB (+87.5% compared with control conditions, P<0.005). After treatment with 100 ng ml−1 PDGFB, proliferation decreased compared with treatment with 50 ng ml−1 PDGFB (+71.3% compared with control conditions, P<0.005).
The exposure to VEGFA increased proliferation in meningiomas in vitro: Proliferation was determined on the basis of the malignant meningioma cell line IOMM-Lee (A and B) and two benign meningiomas (C and D). The cells were incubated for 4 h with serum-free medium and fresh growth factor. The control represents proliferation independent of supplemented growth factor. Proliferation was measured using a BrdU assay. (A and C) VEGFA (B and D) PDGFB. (E) The inhibition of PDGFRβ decreased the VEGFA-induced proliferation. The IOMM-Lee cells were pre-treated for 1 h with 10 μg ml−1 of either anti-KDR or -PDGFRβ neutralisation antibodies, 100 nM KDR tyrosine kinase inhibitor (KDR-TK) and 2 μ M PDGFRβ tyrosine kinase inhibitor (PDGFRβ-TK), followed by either 10 ng ml−1 VEGFA or 10 ng ml−1 PDGFB for 4 h. The control represents VEGFA- or PDGFB-induced proliferation. (F) The inhibition of PDGFRβ or KDR decreased VEGFA-induced proliferation in benign meningiomas. After pre-treatment, the cells were incubated with either 50 ng ml−1 VEGFA or 50 ng ml−1 PDGFB. The control represents VEGFA- or PDGFB-induced proliferation. The data shown are the mean number of proliferating cells±s.d. determined from five random fields from each of three independent experiments. *P<0.05; **P<0.005.
To determine if these results also apply to benign meningiomas with low proliferation, two primary cell cultures of meningothelial meningiomas were treated as described. As shown in Figure 3C, the mean proliferation without growth factors was 5.4±1.7%. Cell proliferation was significantly increased after treatment with 20 ng ml−1 VEGFA (+100% compared with control conditions, P<0.05), 50 ng ml−1 VEGFA (+126.2% compared with control conditions, P<0.005), and 100 ng ml−1 VEGFA (+114.7% compared with control conditions, P<0.001). Treatment with PDGFB significantly increased proliferation in benign meningiomas after treatment with 10 ng ml−1 PDGFB (+84.7% compared with control conditions, P<0.05), 20 ng ml−1 PDGFB (+99.1% compared with control conditions, P<0.005), and 50 ng ml−1 PDGFB (+134.4% compared with control conditions, P<0.005). After treatment with 100 ng ml−1 PDGFB proliferation slightly decreased compared with treatment with 50 ng ml−1 PDGFB (+130.8% compared with control conditions, P<0.005), as shown in Figure 3D.
To evaluate the functional effect of these findings, PDGFRβ and KDR were inhibited with an anti-PDGFRβ neutralisation antibody or a PDGFRβ tyrosine kinase inhibitor or an anti-KDR neutralisation antibody or a KDR tyrosine kinase inhibitor, respectively, before stimulation with VEGFA.
Pre-treatment with either 10 μg ml−1 anti-PDGFRβ neutralisation antibody or 2 μ M PDGFRβ tyrosine kinase inhibitor (PDGFRβ-TK) for 1 h followed by incubation with 10 ng ml−1 VEGFA for 4 h resulted in a significant decrease in IOMM-Lee cell growth compared with VEGFA grown without pre-treatment (−38.7% after anti-PDGFRβ pre-treatment, P<0.005 and −32.6% after PDGFRβ-TK pre-treatment, P<0.05, Figure 3E). In contrast, pre-treatment with either 10 μg ml−1 anti-KDR neutralisation antibody or 100 nM KDR tyrosine kinase inhibitor (KDR-TK) before incubation with VEGFA displayed no anti-proliferating effect.
In addition, two benign meningiomas were pre-treated as described before and then incubated with 50 ng ml−1 VEGFA for 4 h, because the primary meningioma cell cultures displayed the highest proliferation effect after treatment with this growth factor concentration. Cell growth significantly decreased after the inhibition of PDGFRβ (−43.1% after anti-PDGFRβ pre-treatment, P<0.05 and −54.2% after PDGFRβ-TK pre-treatment, P<0.05; Figure 3F). The control cells were incubated with 50 ng ml−1 PDGFB. Cell proliferation significantly decreased after anti-PDGFRβ pre-treatment 37.7% and decreased 44.4% after PDGFRβ-TK pre-treatment (P<0.05). In addition, meningioma cell growth significantly decreased after the inhibition of KDR and incubation with VEGFA (−47.5% after anti-KDR pre-treatment, P<0.05 and −48.1% after KDR-TK pre-treatment, P<0.005).
VEGFA induced PDGFRβ tyrosine phosphorylation
To provide further evidence that PDGFRβ is stimulated by VEGFA, six randomly chosen primary meningioma cells (four WHO °I+two WHO °II) were starved for 24 h in serum-free medium. The cells were stimulated with increasing amounts of either VEGFA or PDGFB for 10 min at 37°C. PDGFRβ expression and PDGFRβ phosphorylation were measured with the TaqMan Protein Assay. The HUVEC was used as the control. PDGFRβ and KDR expression was determined in different cell types (Figure 4A). As expected, the HUVECs displayed high KDR and very low PDGFRβ expression. Because meningiomas are of mesenchymal origin cultured normal mesothelial cells (HMCs) were also examined. These cells showed both PDGFRβ and KDR expression. In contrast, HNCs displayed very low KDR expression and decreased PDGFRβ levels compared with the brain.
VEGFA stimulated PDGFRβ tyrosine phosphorylation is comparable to PDGFB in meningiomas in vitro. (A) PDGFRβ and KDR expression was compared between meningiomas, HUVECs, HNCs, and HMCs. The data are expressed as relative quantities (RQs) compared with the brain (RQBrain=0). The HUVECs were used as a PDGFRβ-negative and KDR-positive control. (B) The tyrosine phosphorylation was determined in HUVECs as a control. The HUVECs were starved for 24 h with serum-free medium. The cells were stimulated with increasing concentrations of either VEGFA or PDGFB for 10 min at 37°C. The tyrosine phosphorylation was measured using a TaqMan Protein Assay. The data are shown as the mean number of two independent experiments using different HUVEC cultures. (C and D) Six primary meningioma cell cultures were given the same treatment as the HUVECs. The PDGFRβ expression and PDGFRβ phosphorylation were determined. *P<0.05; #P<0.005, compared with the respective unstimulated cells. The bars represent the mean±s.d.
After the HUVECs were stimulated with VEGFA, the tyrosine phosphorylation of KDR was evaluated. The tyrosine phosphorylation of KDR reached a maximum with 10 ng ml−1 VEGFA and decreased with higher VEGFA concentrations (Figure 4B). The tyrosine phosphorylation level of PDGFRβ was determined after stimulation with PDGFB, which was three-fold higher after stimulation with 10 ng ml−1 PDGFB.
All meningiomas tested displayed high PDGFRβ expression, and KDR was not detectable. The PDGFRβ expression and PDGFRβ phosphorylation were determined from VEGFA-treated primary meningioma cells. As shown in Figure 4C, the phosphorylation of PDGFRβ by VEGFA increased with increasing concentrations. The tyrosine phosphorylation level was three-fold higher after stimulation with 10 ng ml−1 VEGFA (fold change=3.4±1.18, P<0.05) and doubled after stimulation with 20 ng ml−1 VEGFA (fold change=6.1±3.62, P<0.05). The tyrosine phosphorylation of PDGFRβ reached its maximum with 100 ng ml−1 VEGFA (fold change=9.5±4.79, P<0.005). The control cells were also stimulated with PDGFB. As shown in Figure 4D, the tyrosine phosphorylation of PDGFRβ increased higher after stimulation with 5 ng ml−1 PDGFB (fold change=3.1±1.75, P<0.05) and 10 ng ml−1 PDGFB (fold change=5.5±1.28, P<0.005). After stimulation with 20 ng ml−1 PDGFB, the tyrosine phosphorylation of PDGFRβ was similar to VEGFA (fold change=6.9±0.98, P<0.005). With 50 ng ml−1 PDGFB, the tyrosine phosphorylation of PDGFRβ reached its maximum (fold change=11.9±3.43, P<0.005).
Gambogic acid, sunitinib, and tandutinib inhibit migration of primary meningioma cells
The Radius cell migration assay was used to examine the migration of six randomly chosen primary meningioma cells (four WHO °I+two WHO °II) after treatment with the angiogenesis inhibitors sunitinib and tandutinib. Sunitinib is a tyrosine kinase inhibitor that inhibits at least eight receptor protein tyrosine kinases, including KDR and PDGFRβ, at the same IC50 (10 nM) (Mendel et al, 2003). In contrast, tandutinib is a more selective tyrosine kinase inhibitor that does not inhibit KDR up to IC50=30 μ M but affects PDGFRβ in vitro at IC50=30 nM and in cellular assays at IC50=200 nM (Kelly et al, 2002). The effect of gambogic acid on meningioma cell migration was also examined. Gambogic acid inhibits the transferrin receptor with an IC50 of 1 μ M, induces apoptosis and inhibits cell growth with an EC50 of 0.5–1.5 μ M (Kasibhatla et al, 2005).
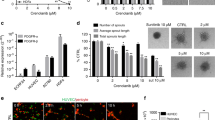
Sunitinib, tandutinib, and gambogic acid suppressed cell migration (Figure 5A). With increasing doses of gambogic acid (b–i), meningioma cells became unable to close the uncoated gap in 24 h. Figure 5B shows the dose-dependent inhibition of cell migration by meningiomas following incubation with 31.25–2000 ng ml−1 gambogic acid. Cell migration was inhibited in a concentration-dependent manner with a mean of 9.3% for 31.25 ng ml−1 gambogic acid to 72.1% for 2000, ng ml−1 gambogic acid. Migration was suppressed by 50% on average with 1 μg ml−1 gambogic acid (1.6 nM). Sunitinib and tandutinib inhibited cell migration similar to gambogic acid. Migration was suppressed by 50% on average with 3.8 nM sunitinib and with 3.6 nM tandutinib (Supplementary material online).
Gambogic acid inhibits the migration of meningioma cells in vitro. Eight different primary meningioma cells were plated in 24-well plates. The next day, the coating of the gap was removed followed by treatment for 24 h with either gambogic acid or vehicle (DMSO). Each gap was photographed after 24 h, except for the first, which was photographed after 0 h. (A) A representative experiment is shown. (a) The control group after 0 h. (b) The control group after 24 h. (c) Treatment with 31.25 ng ml−1 gambogic acid. (d) Treatment with 62.5 ng ml−1 gambogic acid. (e) Treatment with 125 ng ml−1 gambogic acid. (f) Treatment with 250 ng ml−1 gambogic acid. (g) Treatment with 500 ng ml−1 gambogic acid. (h) Treatment with 1000 ng ml−1 gambogic acid. (i) Treatment with 2000, ng ml−1 gambogic acid. (B) The concentration-dependent effect of gambogic acid on primary meningioma cells in a gap assay. The pictures were analysed using CellProfiler. The data are shown as the mean number of the inhibitory effect of gambogic acid compared with the uninhibited meningioma cells. (C) The primary meningioma cells were pre-treated with increasing concentrations of gambogic acid for 1 h. Then, the cells were stimulated with 100 ng ml−1 VEGFA for 10 min at 37°C. The PDGFRβ expression and PDGFRβ phosphorylation were measured using a TaqMan Protein Assay. (B and C) *P<0.05, compared with the respective uninhibited cells. The bars represent the mean±s.d.
Gambogic acid inhibits tyrosine phosphorylation of PDGFRβ
Gambogic acid inhibits cell migration by suppressing PDGFRβ tyrosine phosphorylation, as demonstrated by Liu et al (2010). Thus, three randomly chosen primary meningothelial meningioma cells were pre-treated with increasing concentrations (0.125–2 ng ml−1) of gambogic acid for 1 h. Then, cells were stimulated with either 100 ng ml−1 VEGFA or PDGFB for 10 min at 37°C. PDGFRβ expression and PDGFRβ phosphorylation were measured using a TaqMan Protein Assay. As shown in Figure 5C, gambogic acid inhibited the tyrosine phosphorylation of PDGFRβ after stimulation with VEGFA and PDGFB (data not shown) in a concentration-independent manner (−67% on average compared with uninhibited cells, P<0.05).
Discussion
In this study, we demonstrated that VEGFA signals through PDGFRβ in meningioma cells in vitro. We measured the RNA and protein levels of angiogenesis-related genes with sensitive and novel techniques. The KDR was barely detectable in meningiomas with either qRT–PCR or the TaqMan Protein Assay. In contrast, PDGFRβ was significantly upregulated in meningiomas compared with normal human brain samples. To provide evidence that VEGFA signalling through PDGFRβ has biological significance, we compared the effects of VEGFA and PDGFB on cell proliferation. Additionally, we demonstrated that stimulation with VEGFA induced PDGFRβ tyrosine phosphorylation in meningiomas similar to PDGFB-stimulated PDGFRβ. We also noted that the PDGFRβ inhibitors sunitinib, tandutinib, and gambogic acid inhibit the migration of meningioma cells in vitro.
Meningiomas are highly vascularised tumours. Several studies have demonstrated the significance of VEGF in the proliferation and migration of meningiomas in which VEGF has been upregulated (Provias and Claffey, 1997; Christov et al, 1999; Lamszuz et al, 2000; Park et al, 2000; Yamasaki, 2000). Some groups have observed a correlation between the degree of VEGF expression and tumour vascularity or invasiveness (Samoto et al, 1995; Provias and Claffey, 1997). In contrast, Pietsch et al (1997) and Nishikawa et al (1998) could not find a relationship between VEGF expression and tumour vasculature (Pietsch et al, 1997; Nishikawa et al, 1998). Lamszuz et al (2000) noted a strong correlation between meningioma histological grade and VEGF expression, wherein increased VEGF expression was linked to higher WHO grades. However, Barresi and Tuccari (2010) showed the absence of correlation between VEGF expression and WHO grade (Barresi and Tuccari, 2010). Another study correlated VEGF expression and the recurrence of benign meningiomas (Yamasaki, 2000).
Uesaka et al (2007) quantified VEGFA expression in meningiomas compared with normal human brain samples using qRT–PCR and did not observe any changes in expression (Uesaka et al, 2007). In contrast, we observed that VEGFA RNA levels in vitro and in vivo were significantly increased in meningiomas compared with the brain but were downregulated compared with the arachnoidea and dura. The VEGFA expression in vitro and in vivo did not differ between WHO grades. Only the immunohistochemical staining of VEGFA showed increased levels in WHO °III meningiomas. This result was not confirmed by qRT–PCR or the Taqman protein assay results. Due to the low sample numbers of anaplastic meningiomas results can be biased especially when using only a single method. While VEGFA was upregulated on average, VEGFA expression in meningiomas displayed a broad distribution especially in vivo, which may explain opposing observations.
Several studies have examined KDR and FLT1 in meningiomas (Hatva et al, 1995; Otsuka et al, 2004; Huang et al, 2005; Preusser et al, 2012). Hatva et al (1995), Otsuka et al (2004), and Preusser et al (2012) determined KDR expression using either immunohistochemistry or in situ hybridisation. Hatva et al (1995) examined only three meningiomas, which cannot provide justifiable conclusions. The KDR protein expression was detected in 31.4% of specimens (Otsuka et al, 2004), and KDR mRNA was demonstrated in 44.4% of samples (Preusser et al, 2012). Those results show that KDR is not expressed in the majority of meningiomas. The data also provide no information about the expression level of KDR. Our finding that most examined meningiomas displayed no KDR protein expression does not contradict previous studies. In addition, the methods used may influence the results. Our immunohistochemical data for KDR expression show 87% positive specimens, but most meningiomas displayed low KDR levels.
Contradicting results were reported by Huang et al (2005), who measured KDR and FLT-1 expression in various brain tumours with qRT–PCR, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to normalise and compare the results with those in HUVECs. Of the brain tumours that were examined, meningiomas had the highest KDR and FLT-1 levels. In contrast, we determined very low KDR RNA expression in most examined meningiomas.
Huang et al (2005) also observed higher VEGF receptor levels in meningiomas compared with the brain. Our data showed that KDR expression was significantly decreased in meningiomas compared with the brain. These RNA findings were verified by measuring KDR with a TaqMan Protein Assay. The reason for these contradictory results cannot be satisfactorily explained because the study does not state the number of examined samples. A few meningiomas show higher KDR expression compared with the brain. If a small specimen number was analysed, then that could account for the high KDR RNA levels.
Low or absent KDR expression suggests that mechanisms other than VEGFA signalling through KDR influence angiogenesis in meningiomas.
In addition to VEGF, PDGF signalling indirectly regulates angiogenesis. Yang (2001) showed that nearly all meningiomas express PDGFB and PDGFRβ in vitro, and both were increased in WHO °II meningiomas. Several groups have proposed an autocrine mechanism in meningiomas, based on the co-expression of PDGFB and PDGFRβ, which regulate growth (Wang et al, 1990; Figarella-Branger et al, 1994; Kuratsu et al, 1994; Mauro et al, 1995).
In contrast, we showed that PDGFB expression in vitro and in vivo was significantly decreased compared with the brain and dura. Further, PDGFRβ expression in vivo and in vitro rose in meningiomas compared with the brain. The significantly decreased in vitro PDGFRβ RNA levels in WHO °III meningiomas were probably influenced by the small sample numbers (n=5). The low number is due to their rarity among meningiomas. Moreover, surgically resected anaplastic meningiomas are almost always previously irradiated, which influences the cell quality.
High PDGFRβ expression combined with low KDR and PDGFB levels in meningiomas suggests that PDGFRβ has a significant function in tumorigenesis in meningiomas.
We determined that the addition of exogenous VEGFA doubled cell proliferation in malignant IOMM-Lee and benign primary meningioma cells, comparable to exogenous PDGFB, suggesting that VEGFA signalling through PDGFRβ is biologically significant in meningiomas. To evaluate the functional effect of these findings, PDGFRβ was inhibited with an anti-PDGFRβ neutralisation antibody or a PDGFRβ tyrosine kinase inhibitor before stimulation with VEGFA. The proliferative stimuli were abolished under these conditions. The control experiments with PDGFB stimulation showed that PDGFRβ inhibitors also neutralised PDGFB-induced proliferation. Why the presence of the PDGFRβ inhibitor did not significantly influence the PDGFB-induced proliferation rate in IOMM-Lee cells remains to be determined.
The cells were also inhibited with an anti-KDR neutralisation antibody or a KDR tyrosine kinase inhibitor before stimulation with VEGFA or PDGFB, respectively. As expected, the PDGFB-induced proliferation was not influenced by KDR inhibition. The VEGFA-induced proliferative stimuli were abolished after KDR inhibition in benign meningiomas but not in IOMM-Lee cells. There are two possible ways to interpret these results. First, the VEGFA-induced proliferative stimuli may be dependent on the binding of VEGFA to PDGFRβ as implied by the IOMM-Lee data. Second, the VEGFA-induced cell proliferation may rely on both KDR and PDGFRβ binding to VEGFA. Because some meningiomas express detectable KDR the second possibility is preferable to the first.
To further investigate the effect of VEGFA on PDGFRβ in meningiomas, we determined whether VEGFA caused the tyrosine phosphorylation of PDGFRβ. The protein lysates from VEGFA-treated primary meningioma cells were analysed with a TaqMan Protein Assay, which allowed for the quantification of tyrosine phosphorylation in meningiomas. We demonstrated that VEGFA induces concentration-dependent PDGFRβ tyrosine phosphorylation in meningiomas after 10 min. A comparison with PDGFB-induced meningioma cells showed a similar increase in the phosphorylation of PDGFRβ. The effect of VEGFA on KDR was not investigated because KDR was not detectable in the meningiomas used. Thus, the data suggest that VEGFA mediates proliferation and migration through PDGFRβ, but not KDR, in meningiomas.
To determine if selective PDGFRβ inhibitors, such as tandutinib, have a similar effect as sunitinib, which also inhibits KDR and several other receptor protein tyrosine kinases, we treated primary meningioma cells with these two tyrosine kinase inhibitors. Tandutinib suppressed cell migration in primary meningiomas as effectively as sunitinib, in spite of the fact that tandutinib only inhibits PDGFRβ.
We also investigated the effect of gambogic acid on primary meningiomas. Recently Liu et al (2010) reported that gambogic acid inhibits cell migration by suppressing PDGFRβ tyrosine phosphorylation. The treatment of meningiomas in vitro with gambogic acid significantly inhibited cell migration. Furthermore, gambogic acid inhibited the tyrosine phosphorylation of PDGFRβ after stimulation with VEGFA, independent of the gambogic acid concentration. These results support those of Liu et al (2010), showing that gambogic acid inhibits cell migration by suppressing PDGFRβ tyrosine phosphorylation.
The low expression or absence of KDR in meningiomas both in vitro and in vivo, combined with the elevated level of PDGFRβ, suggests that VEGFA primarily regulates VEGF-mediated migration and proliferation through PDGFRβ, not KDR, in meningiomas. This model is supported by our findings in which the stimulation of meningiomas in vitro with VEGFA upregulates cell proliferation and induces PDGFRβ tyrosine. Gambogic acid, tandutinib, and sunitinib were equally potent at inhibiting meningioma growth in vitro, suggesting that selective PDGFRβ inhibitors, such as gambogic acid and tandutinib, should be evaluated as potential therapies for recurrent and malignant meningiomas, possibly in combination with VEGF inhibitors.
Change history
26 October 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Barresi V, Tuccari G (2010) Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology 30 (5): 537–546
Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM (2006) CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100
Cheng Y, Paz K (2008) Tandutinib, an oral, small-molecule inhibitor of FLT3 for the treatment of AML and other cancer indications. IDrugs 11 (1): 46–56
Christov C, Lechapt-Zalcman E, Adle-Biassette H, Nachev S, Gherardi R (1999) Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptor flt-1 in microcystic meningiomas. Acta Neuropathol 98: 414–420
Cross M, Dixelius J, Matsumoto T, Claesson-Welsh L (2003) VEGF receptor signal transduction. Trends Biochem Sci 28: 488–494
Erickson HS, Albert PS, Gillespie JW, Wallis BS, Rodriguez-Canales J, Linehan WM, Gonzalez S, Velasco A, Chuaqui RF, Emmert-Buck MR (2007) Assessment of normalization strategies for quantitative RT-PCR using microdissected tissue samples. Lab Invest 87 (9): 951–962
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9 (6): 669–676
Figarella–Branger D, Vagner–Capodano AM, Bouillot P, Graziani N, Gambarelli D, Devictor B, Zattara–Cannoni H, Bianco N, Grisoli F, Pellissier JF (1994) Platelet-derived growth factor (PDGF) and receptor (PDGFR) expression in human meningiomas: correlations with clinicopathological features and cytogenetic analysis. Neuropathol Appl Neurobiol 20 (5): 439–447
Gu H, Wang X, Rao S, Wang J, Zhao J, Ren FL, Mu R, Yang Y, Qi Q, Liu W, Lu N, Ling H, You Q, Guo Q (2008) Gambogic acid mediates apoptosis as a p53 inducer through down-regulation of mdm2 in wild-type p53-expressing cancer cells. Mol Cancer Ther 7 (10): 3298–3305
Hatva E, Kaipainen A, Mentula P, Jaaskelainen J, Paetau A, Haltia M, Alitalo K (1995) Expression of endothelial cell-specific receptor tyrosine kinases and growth factors in human brain tumors. Am J Pathol 146: 368–378
Holmes DI, Zachary I (2005) The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 6: 209–219
Huang H, Held-Feindt J, Buhl R, Mehdor nH, Mentlein R (2005) Expression of VEGF and its receptors in different brain tumors. Neurol Res 27: 371–377
Jääskeläinen J (1986) Seemingly complete removal of histologically benign intracranial meningioma: Late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 26 (5): 461–469
Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, Qiu L, Archer SP, Ponce AE, Sirisoma N, Jiang S, Zhang H-Z, Gehlsen KR, Cai SX, Green DR, Tseng B (2005) A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci USA 102 (34): 12095–12100
Kelly LM, Yu J-C, Boulton CL, Apatira M, Li J, Sullivan CM, Williams I, Amaral SM, Curley DP, Duclos N, Neuberg D, Scarborough RM, Pandey A, Hollenbach S, Abe K, Lokker NA, Gilliland DG, Giese NA (2002) CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Cancer Cell 1 (5): 421–432
Kuratsu JI, Seto H, Kochi M, Ushio Y (1994) Expression of PDGF, PDGF-receptor, EGF-receptor and sex hormone receptors on meningioma. Acta Neurochir (Wien) 131 (3): 289–293
Lamszuz K, Lengler U, Schmidt NO, Stavrou D, Ergun S, Westphal M (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46: 938–947
Liu Y, Li W, Ye C, Lin Y, Cheang T-Y, Wang M, Zhang H, Wang S, Zhang L, Wang S (2010) Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor β tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells. J Atheroscler Thromb 17 (9): 901–913
Louis D, Ohgaki H, Wiestler O, Cavenee W, Burger P, Jouvet A, Scheithauer B, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114 (2): 97–109
Mahmood A, Caccamo DV, Tomecek FJ, Malik GM (1993) Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery 33 (6): 955–963
Mauro A, Di Sapio A, Mocellini C, Schiffer D (1995) Control of meningioma cell growth by platelet-derived growth factor (PDGF). J Neurol Sci 131 (2): 135–143
McMullen KP, Stieber VW (2004) Meningioma: current treatment options and future directions. Curr Treat Options Oncol 5 (6): 499–509
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JÖ, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors. Clin Cancer Res 9 (1): 327–337
Motzer RJ, Hoosen S, Bello CL, Christensen JG (2006) Sunitinib malate for the treatment of solid tumours: a review of current clinical data. Expert Opin Investig Drugs 15 (5): 553–561
Nakasu S, Fukami T, Jito J, Nozaki K (2009) Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol 26 (2): 69–72
Nishikawa R, Cheng S, Nagashima R, Huang H, Cavenee W, Matsutani M (1998) Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol 96: 453–462
Otsuka S, Tamiya T, Ono Y, Michiue H, Kurozumi K, Daido S, Kambara H, Date I, Ohmoto T (2004) The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol 70 (3): 349–357
Park K, Kim JH, Nam DH, Lee JI, Kim JS, Hong SC, Shin HJ, Eoh W, Park K (2000) Vascular endothelial growth factor expression under ischemic stress in human meninigiomas. Neurosci Lett 283: 45–48
Pfister C, Tatagiba MS, Roser F (2011) Selection of suitable reference genes for quantitative real-time polymerase chain reaction in human meningiomas and arachnoidea. BMC Res Notes 4: 275
Pietsch T, Valter MM, Wolf HK, von Deimling A, Huang HJ, Cavenee WK, Wiestler OD (1997) Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol 93: 109–117
Preusser M, Hassler M, Birner P, Rudas M, Acker T, Plate KH, Widhalm G, Knosp E, Breitschopf H, Berger J, Marosi C (2012) Microvascularization and expression of VEGF and its receptors in recurring meningiomas: pathobiological data in favor of anti-angiogenic therapy approaches. Clin Neuropathol 31: 352–360
Provias J, Claffey K, delAguila L, Lau N, Feldkamp M, Guha A (1997) Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery 40: 1016–1026
Qiang L, Yang Y, You Q-D, Ma Y-J, Yang L, Nie F-F, Gu H-Y, Zhao L, Lu N, Qi Q, Liu W, Wang X-T, Guo Q-L (2008) Inhibition of glioblastoma growth and angiogenesis by gambogic acid: an in vitro and in vivo study. Biochem Pharmacol 75 (5): 1083–1092
Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5 (12): 1045–1054
Samoto K, Ikezaki K, Ono M, Shono T, Kohno K, Kuwano M, Fukui M (1995) Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res 55: 1189–1193
Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Bornemann A (2006) Patterns of SPARC expression and basement membrane intactness at the tumour-brain border of invasive meningiomas. Neuropathol Appl Neurobiol 32 (5): 525–531
Uesaka T, Shono T, Suzuki SO, Nakamizo A, Niiro H, Mizoguchi M, Iwaki T, Sasaki T (2007) Expression of VEGF and its receptor genes in intracranial schwannomas. J Neurooncol 83: 259–266
Wang J, Nister M, Hermansson M, Westermark B, Ponten J (1990) Expression of PDGF beta-receptors in human meningioma cells. Int J Cancer 46: 772–778
Yamasaki F (2000) Recurrence of meningiomas. Cancer 89: 1102–1110
Yamazaki Y, Morita T (2006) Molecular and functional diversity of vascular endothelial growth factors. Mol Divers 10: 515–527
Yancopoulos GD, Davis S, Gale N, Rudge J, Wiegand S, Holash J (2000) Vascular specific growth factors and blood vessel formation. Nature 407: 242–248
Yang S (2001) Expression of PDGF and its receptor as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J Clin Neurosci 8 (4): 49–53
Yu J, Ustach C, Kim H (2003) Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol 36: 49–59
Acknowledgements
This study was supported by grants from the Else Uebelmesser-Stiftung. We are grateful to Anita Lal (UCSF, USA) for kindly providing the IOMM-Lee cell line.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Pfister, C., Pfrommer, H., Tatagiba, M. et al. Vascular endothelial growth factor signals through platelet-derived growth factor receptor β in meningiomas in vitro. Br J Cancer 107, 1702–1713 (2012). https://doi.org/10.1038/bjc.2012.459
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.459
Keywords
This article is cited by
-
Drug target therapy and emerging clinical relevance of exosomes in meningeal tumors
Molecular and Cellular Biochemistry (2024)
-
Glucose degradation products in peritoneal dialysis solution impair angiogenesis by dysregulating angiogenetic factors in endothelial and vascular smooth muscle cells
Clinical and Experimental Nephrology (2022)
-
Extracellular Vesicles in Essential Hypertension: Hidden Messengers
Current Hypertension Reports (2020)
-
Intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT
Acta Neuropathologica Communications (2019)
-
SSTR-2 as a potential tumour-specific marker for fluorescence-guided meningioma surgery
Acta Neurochirurgica (2018)