Abstract
The use of trastuzumab, a monoclonal antibody that targets the human epidermal growth factor receptor 2 (HER2) alteration present in 25 to 30% of breast cancers, has been associated with improved survival outcomes in both the adjuvant and metastatic settings. However, despite the robust clinical efficacy of trastuzumab in HER2-positive metastatic breast cancer (MBC), primary and secondary resistance remains a clinical challenge. Although lapatinib has demonstrated modest activity in this setting, trials reported to date have yet to demonstrate improvements in overall survival with its use. Novel therapeutic strategies to circumvent trastuzumab resistance are warranted, and agents targeting the HER, vascular endothelial growth factor, heat shock protein 90, phosphoinositide 3 kinase/Akt/mammalian target of rapamycin, and insulin-like growth factor-1 receptor pathways represent rational approaches in the management of HER2-positive disease. In this review, early-phase and emerging trial data surrounding the use of these promising agents in HER2-positive MBC will be discussed.
Similar content being viewed by others
Main
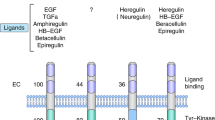
The human epidermal growth factor receptor 2 (HER2) oncogene is amplified and overexpressed in 25 to 30% of breast cancers and has provided a basis for targeted therapeutics of this originally poor-prognostic phenotype (Slamon et al, 1987; Slamon et al, 1989). Amplification of HER2 (ErbB2), a member of the ErbB/HER family of receptor tyrosine kinases that also include epidermal growth factor receptor (EGFR)/HER1/ErbB1, HER3/ErbB3 and HER4/ErbB4, leads to increased receptor homo- and hetero-dimerisation and subsequent activation of downstream signaling pathways associated with cell proliferation, differentiation, survival and angiogenesis (Yarden and Sliwkowski, 2001). Trastuzumab (Herceptin; Genentech, South San Francisco, CA, USA), the first available HER2-directed therapy, is a humanised monoclonal antibody that targets the HER2 extracellular domain, and was approved by the US Food and Drug Administration in 1998 for the management of HER2-positive metastatic breast cancer (MBC) in combination with chemotherapy. The anti-proliferative and cytotoxic effects of trastuzumab likely result from a combination of antibody-dependent cellular cytotoxicity, inhibition of extracellular domain cleavage, decreased DNA repair, decreased intracellular signal transduction and anti-angiogenic effects (Spector and Blackwell, 2009). The pivotal phase-III trial of trastuzumab plus chemotherapy in the first-line management of HER2-positive MBC demonstrated robust improvements in response rates (RRs; 50% vs 32%), median time to progression (TTP; 7.4 vs 4.6 months) and median overall survival (25 vs 20 months) with the addition of trastuzumab (Slamon et al, 2001). Nonetheless, primary and secondary resistance are frequently encountered. Possible mechanisms include HER2 crosstalk with other ErbB members or insulin-like growth factor-1 receptor (IGF-1R) (Nahta et al, 2004), presence of p95-HER2, a truncated receptor that lacks the extracellular binding domain for trastuzumab (Scaltriti et al, 2007), PTEN deficiency (phosphatase and tensin homologue deleted on chromosome 10) (Nagata et al, 2004), increased phosphoinositide 3 kinase (PI3k)/Akt pathway activation (Berns et al, 2007) and, more recently, presence of Rac1, a Ras-like small GTPase that affects trastuzumab-mediated endocytosis of the ErbB2 receptor (Dokmanovic et al, 2009). Still, no one mechanism has been defined in the clinic and it is not clear that resistance to trastuzumab means a complete loss of dependence on HER2 signaling.
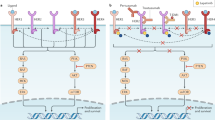
Developing novel targeted agents for use in HER2-positive breast cancer remains clinically significant. In this review, strategies to overcome resistance to trastuzumab therapy (Figure 1) will be discussed including novel antibody-based approaches against HER2, newer ErbB-family tyrosine kinase inhibitors (TKIs), anti-angiogenic therapies, heat shock protein 90 (hsp90) inhibitors, PI3K and mammalian target of rapamycin (mTOR) inhibitors, and IGF-1R inhibitors (Tables 1 and 2).
Targeted agents for HER2-positive MBC. Diagram depicting the molecular targets of approved and investigational agents for HER2-positive MBC. Abbreviations: Akt, protein kinase B; c-kit, stem cell factor receptor; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; hsp90, heat shock protein 90; IGF-1R, insulin-like growth factor-1 receptor; MBC, metastatic breast cancer; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide-3-kinase; RTK, receptor tyrosine kinase; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
New antibody-based targeting of HER2 – pertuzumab, trastuzumab DM1 (T-DM1)
Pertuzumab
Like trastuzumab, pertuzumab (Omnitarg, 2C4; Genentech) targets the HER2 extracellular domain but at a different epitope, resulting in inhibited dimerisation of HER2 with other HER family receptors (EGFR/HER1, HER3 and HER4) (Agus et al, 2002). Preclinical studies in HER2-positive breast cancer have demonstrated promising antitumour efficacy with associated downregulation of PI3K/Akt and MAPK signaling pathways both as a single-agent and synergistically with trastuzumab (Nahta et al, 2004; Agus et al, 2002; Franklin et al, 2004; Scheuer et al, 2009). In a single-arm, phase-II study of trastuzumab plus pertuzumab (every 3 weeks) in 11 patients with HER2-positive MBC and disease progression on trastuzumab, an objective RR of 18% was reported, with partial responses in 2 patients and stable disease in 3 patients (Portera et al, 2008). The median TTP was 6 weeks. Using a lower limit left ventricular ejection fraction (LVEF) cut-off of 55%, six cases of left ventricular systolic dysfunction were seen (three grade 1, two grade 2 and one grade 3) in this trial, raising concerns over cardiotoxicity (Portera et al, 2008). In another single-arm phase-II trial of trastuzumab (weekly or every 3 weeks (q3 weekly)) plus pertuzumab (every 3 weeks) in 66 patients with trastuzumab-refractory, HER2-positive MBC, a similar objective RR was also demonstrated at 24.2%, including 5 patients with complete response (7.6%) and 11 with partial response (16.7%) (Baselga et al, 2010). Furthermore, stable disease ⩾6 months was seen in an additional 17 patients (25.8%), yielding a 50% clinical benefit rate (CBR). The median progression-free survival (PFS) was 5.5 months in this cohort. In contrast to the previous phase-II study, cardiotoxicity was less of an issue despite the use of identical drug doses in the q3 weekly regimen, with only three patients experiencing an asymptomatic LVEF decline ⩾10% and an absolute LVEF <50%. In two out of the three patients, recovery in LVEF was seen without treatment interruption, whereas the third patient withdrew from the study because of progressive disease. Overall, the trastuzumab/pertuzumab combination was well tolerated, with diarrhoea (64%), fatigue (33%), nausea (27%) and rash (26%) being the most common toxicities (Baselga et al, 2010). Ongoing trials are currently evaluating combinations of pertuzumab with trastuzumab and chemotherapy in HER2-positive MBC, including a phase-II trial of trastuzumab/capecitabine±pertuzumab in the second-line setting (PHEREXA; NCT01026142) and a large global phase-III trial of trastuzumab/docetaxel±pertuzumab in the first-line setting (CLEOPATRA; NCT00567190). Combinations of pertuzumab with T-DM1 (discussed below) are also under investigation in multiple phase-I–III studies.
T-DM1
T-DM1 (Genentech) represents a novel approach to drug delivery in which the monoclonal antibody trastuzumab is conjugated to an anti-microtubule agent (emtansine) (Lewis Phillips et al, 2008; Junttila et al, 2010). In preclinical models, potent antitumour activity was observed with T-DM1, including in trastuzumab- and lapatinib-resistant states (Lewis Phillips et al, 2008; Junttila et al, 2010). In a phase-I study of T-DM1 in a heavily pretreated population of HER2-positive MBC in which patients had received a median of four prior systemic agents, an encouraging clinical RR of 44% was reported (Krop et al, 2010). At a maximum tolerated dose of 3.6 mg kg−1 IV q3 weekly, T-DM1 was well-tolerated overall with only mild toxicities (thrombocytopenia, transaminitis, fatigue, nausea and anemia) and no reported cardiotoxicities. In two phase-II trials of single-agent T-DM1 in a heavily pretreated population, impressive RRs were similarly reported (Krop et al, 2009b; Burris et al, 2010). The first trial enrolled 112 patients with HER2-positive MBC who had received a median of three prior chemotherapy agents; the overall RR was 25.9%, and 24.2% in those who had previously received both trastuzumab and lapatinib (Burris et al, 2010). T-DM1 was well tolerated, with grade 3 or 4 hypokalemia (8.9%) and thrombocytopenia (8%) being the most common. The second trial enrolled 110 patients who had previously received a median of seven prior systemic agents (including an anthracycline, taxane, capecitabine, trastuzumab and lapatinib); an objective RR of 32.7% and a CBR of 44.5% were reported (Krop et al, 2009a). Good patient tolerability to T-DM1 was seen, with thrombocytopenia, fatigue, and nausea being the most common adverse events (AEs), and no dose-limiting cardiotoxicities. Recently, preliminary results from a randomised phase-II trial of T-DM1 vs trastuzumab/docetaxel in first-line, HER2-positive MBC were presented (Perez et al, 2010). In 137 patients with a median follow-up of 6 months, single-agent T-DM1 achieved an objective RR of 47.8%, as compared with 41.4% for the trastuzumab/docetaxel arm. A more favorable safety profile was observed with the T-DM1 arm, with a lower incidence of grade 3 and 4 AEs (37.3% vs 75.0%). A recent update of these data also demonstrated a significant increase in investigator-reported PFS with T-DM1 compared with the control arm (14.2 vs 9.2 months, respectively (Hurvitz et al, 2011). Preliminary data from a single-arm phase-Ib/II trial evaluating the combination of pertuzumab and T-DM1 in patients with previously untreated (n=21) and relapsed (n=46) HER2-positive MBC showed a RR of 57.1% in previously untreated patients (majority had received trastuzumab (86%), taxanes (71%) and anthracyclines (62%) in the adjuvant setting) and a RR of 34.8% in patients with relapsed disease (Dieras et al, 2010). T-DM1 plus pertuzumab appeared to be well-tolerated overall, although cardiotoxicity was observed with LVEF declines in two patients. The results of large global phase-III trials of T-DM1, including T-DM1 vs lapatinib plus capecitabine in patients previously treated with a taxane and trastuzumab (EMILIA; NCT00829166), as well as a three-arm trial evaluating T-DM1 vs T-DM1/pertuzumab vs trastuzumab/taxane in the first-line setting (MARIANNE; NCT01120184), are eagerly awaited.
HER-family TKIs – lapatinib, neratinib and afatinib
Lapatinib
Lapatinib (Tykerb; GlaxoSmithKline, London, UK) is a small molecule, reversible, dual inhibitor of EGFR/HER1 and HER2, currently approved by the US Food and Drug Administration for use in MBC. Preclinical studies demonstrated potent antitumour effects in HER2-overexpressing models, including in cell lines with acquired trastuzumab resistance (Rusnak et al, 2001; Xia et al, 2002; Konecny et al, 2006). In phase-I and -II trials of single-agent lapatinib in patients with HER2-positive breast cancer refractory to trastuzumab, lapatinib exhibited modest clinical activity and a tolerable toxicity profile (diarrhoea and rash) (Burris et al, 2005; Burstein et al, 2008b; Blackwell et al, 2009). The pivotal phase-III trial evaluated the combination of lapatinib (1250 mg daily) plus capecitabine (2000 mg m−2 daily, given on days 1–14 of a 21-day cycle) vs capecitabine alone in patients with HER2-positive locally advanced or MBC who were treatment refractory to an anthracycline, taxane and trastuzumab (Geyer et al, 2006). In this trial of 324 patients, lapatinib plus capecitabine resulted in a 4-month improvement in median TTP (8.4 vs 4.4 months; hazard ratio (HR)=0.49; P<0.001). A higher RR also favoured the combination arm (22% vs 14%), although this was not statistically significant. In the updated efficacy analyses, the improvement in median TTP was confirmed (6.2 vs 4.3 months; HR=0.57; P=0.00013), although no statistical differences in overall survival were demonstrated (Cameron et al, 2008; Cameron et al, 2010). Lapatinib was reasonably well tolerated, with an increased incidence of diarrhoea and rash with the addition of capecitabine. The EGF30008 trial (Johnston et al, 2009) was a phase-III trial that evaluated letrozole plus lapatinib (n=642) vs letrozole plus placebo (n=644) in treatment-naive post-menopausal patients with hormone receptor-positive MBC. Among the 219 estrogen receptor-positive, HER2-positive patients, a 5.2-month improvement in the primary endpoint of median PFS was seen in the lapatinib/letrozole arm (8.2 vs 3.0 months; HR=0.71; P=0.019). Overall survival data are awaited. Interestingly, a biomarker analysis of this study suggests there may be a role for HER-family targeting with letrozole in HER2-negative ER low-expressing tumours (Finn et al, 2009a). In the EGF30001 phase-III trial, which evaluated lapatinib plus paclitaxel vs paclitaxel alone in the first-line setting, a median TTP improvement of 11.3 weeks was observed in the HER2-positive population (36.4 vs 25.1 weeks; HR=0.53), albeit on a subset analysis (Di Leo et al, 2008; Finn et al, 2009b). Total HER2 blockade with lapatinib and trastuzumab was also evaluated in a phase-III trial of lapatinib plus trastuzumab vs lapatinib alone in patients with MBC who had received a median of three prior trastuzumab-containing regimens, and an almost 4-week improvement in PFS (12.0 vs 8.1 weeks; HR=0.73; P=0.008), as well as a doubling of CBR (24.7% vs 12.4%; P=0.01), were observed (Blackwell et al, 2010). Importantly, data thus far only suggest a trend to overall survival improvement (51.6 vs 39.0 weeks; HR=0.75; P=0.106). Interim results of a phase-II trial evaluating the combination of lapatinib and trastuzumab in patients with HER2-positive MBC (cohort 1 (n=40): no prior lapatinib, trastuzumab or chemotherapy for metastatic disease and >1 year since adjuvant trastuzumab, if received; cohort 2 (n=47): one to two prior lines of chemotherapy, including trastuzumab, or relapse within 1 year of adjuvant trastuzumab) were recently presented and showed objective RRs of 41.7% and 25% in cohorts 1 and 2, respectively (Lin et al, 2011). Grade 3/4 treatment-related toxicities were described as uncommon, with grade 3 diarrhoea reported in 7% and all others (not specified) in <3% of patients.
Neratinib
Neratinib (HKI-272; Pfizer, New York, NY, USA) is an irreversible, oral small-molecule TKI of EGFR/HER1, HER2 and HER4 (Rabindran et al, 2004). In preclinical HER2 models, anti-proliferative effects were accompanied by G1 cell-cycle arrest and decreased downstream signal transduction (Rabindran et al, 2004). In a phase-I study of neratinib in advanced solid malignancies, partial response was seen in 8 out of 25 (32%) HER2-positive breast cancer patients who were previously treated with trastuzumab, anthracyclines and taxanes (Wong et al, 2009). Diarrhoea was the dose-limiting toxicity at a maximum tolerated dose of 320 mg once daily. An open-label, phase-II multicenter trial of single-agent neratinib in advanced HER2-positive breast cancer, which enrolled both trastuzumab-refractory (n=66) and trastuzumab-naive (n=70) patients, demonstrated modest clinical activity in both cohorts (Burstein et al, 2010). Objective RRs of 24% and 56% were seen in the trastuzumab-refractory and trastuzumab-naive groups, respectively, with a median PFS of 22.3 and 39.6 weeks. At a dose of 240 mg once daily, diarrhoea was the most common grade 3/4 AE, occurring in up to 30% of patients and necessitating dose reductions and/or symptomatic management. No cases of grade 3 or 4 cardiotoxicity were observed (Burstein et al, 2010). Currently, studies of single-agent neratinib (neratinib vs lapatinib/capecitabine, NCT00777101) and neratinib combinations (with capecitabine, NCT00741260; trastuzumab, NCT00398567; paclitaxel, NCT00445458; vinorelbine, NCT00706030; and neratinib/paclitaxel vs trastuzumab/paclitaxel, NCT00915018) are under evaluation in HER2-positive MBC. The clinical relevance of neratinib as a ‘pan-HER’ family inhibitor and it being irreversible is yet to be proven.
Afatinib
Afatinib (BIBW 2992; Boehringer Ingelheim, Ingelheim, Germany), an anilino–quinazoline-derived irreversible, oral small-molecule ErbB family TKI (EGFR/HER1, HER2 and HER4), has also demonstrated activity in early-phase trials of advanced solid tumours and trastuzumab-refractory HER2-positive breast cancer (Hickish et al, 2009; Yap et al, 2010; Yamamoto et al, 2011). Preclinical data showed low nanomolar potency for EGFR, HER2 and HER4 kinases (Yamamoto et al, 2011), as well as anti-proliferative effects in HER2-dependent models (Li et al, 2008). In a phase-I trial of 53 patients with advanced solid tumours, antitumour efficacy was reported with continuous once-daily dosing of afatinib, including stable disease for ⩾6 months in 1 breast cancer patient (receptor status and prior therapy not specified) (Yap et al, 2010). Gastrointestinal (diarrhoea and nausea/vomiting) and dermatologic AEs (rash and dry skin), as well as fatigue, were most common. An open-label, single-arm phase-II study of afatinib in 41 patients with HER2-positive MBC following trastuzumab failure demonstrated partial responses in 4 patients and stable disease in 8 patients (maintained for at least four cycles) (Hickish et al, 2009). At a dose of 50 mg once daily, grade 3 rash (9.8%) and diarrhoea (22%) were most common. Recently, a global phase-III trial of afatinib in HER2-positive MBC was initiated (LUX-Breast 1; NCT01125566), which is evaluating vinorelbine/afatinib vs vinorelbine/trastuzumab in patients with prior trastuzumab therapy.
Anti-angiogenic strategies – bevacizumab, sunitinib and pazopanib
Bevacizumab
Preclinical and clinical studies in HER2-positive breast cancer have reported positive associations between HER2 and vascular endothelial growth factor (VEGF) expression levels (Yen et al, 2000; Yang et al, 2002; Konecny et al, 2004). In a phase-II trial evaluating trastuzumab and the monoclonal anti-VEGF antibody bevacizumab (Avastin, Genentech) in the first-line metastatic setting, an objective clinical RR by the World Health Organization criteria of 48% and a CBR of 60% were reported (Hurvitz et al, 2009). Cardiovascular toxicities, including hypertension (most common AE), as well as declines in LVEF (15 grade 1/2, 1 grade 4), and ulcer perforation were seen. Two ongoing phase-III trials, AVEREL (docetaxel/trastuzumab±bevacizumab; NCT00391092) and ECOG 1105 (carboplatin/paclitaxel/trastuzumab±bevacizumab; NCT00520975), are evaluating the addition of bevacizumab to chemotherapy and trastuzumab as first-line therapy in HER2-positive MBC.
In a heavily pre-treated HER2-positive MBC population (median of four prior chemotherapies, three prior biological and two prior hormonal therapies) of which 47 out of 52 (90%) received prior trastuzumab, a phase-II study of lapatinib plus bevacizumab reported a modest overall RR of 13% (Dickler et al, 2008). Diarrhoea and rash, attributable to lapatinib use, were the most common AEs. Declines in LVEF were also observed in this trial (two grade 1 and three grade 2).
VEGFR TKIs
Sunitinib (Sutent; Pfizer) is an oral, multitargeted TKI against VEGFR, platelet-derived growth factor receptor and stem cell factor receptor (c-kit). In an open-label phase-II study of sunitinib monotherapy in patients with MBC previously treated with taxanes and anthracyclines, an overall RR of 11% was observed (Burstein et al, 2008a). However, no correlation between clinical response and ER or HER2 status was found. At present, two early-phase clinical trials are evaluating sunitinib/trastuzumab combinations in HER2-positive breast cancer, including a phase-I trial of sunitinib plus trastuzumab and docetaxel in the first-line setting (NCT00372424) and a phase-II trial of sunitinib plus trastuzumab in the second-line setting (NCT00243503).
Pazopanib (Votrient; GlaxoSmithKline) is an oral multitargeted TKI against VEGFR-1/2/3, platelet-derived growth factor receptor and c-kit. In a randomised phase-II study of pazopanib (400 mg per day) plus lapatinib (1000 mg per day) vs lapatinib alone (1500 mg per day) in HER2-positive, locally advanced or MBC in the first-line setting, an interim analysis of 114 evaluable patients (total n=141) demonstrated modest efficacy with the dual TKI approach (Slamon et al, 2008). Pazopanib plus lapatinib yielded a 12-week progressive disease rate of 15.9% vs 36.8% for lapatinib monotherapy (by investigator assessment). A secondary endpoint of 12-week RR also favoured the combination arm at 44.9% vs 27.8% (by investigator assessment; 36.2% vs 22.2% by independent assessment). AEs of diarrhoea, nausea, transaminitis, hypertension, fatigue and dysgeusia were potentiated with the pazopanib/lapatinib combination, whereas hair color change was solely observed in the dual TKI arm. Notably, four patients experienced declines in LVEF (three asymptomatic and one symptomatic) with the combined anti-HER2/VEGF strategy.
Hsp90 inhibitors
A novel therapeutic approach involves targeting the hsp90 molecular chaperone, whose function includes regulating the stability and maturation of various oncoproteins including HER2 (Trepel et al, 2010). Tanespimycin (17-AAG, KOS-953; Bristol-Myers Squibb, New York, NY, USA), a first-generation geldanamycin derivative, has demonstrated robust antitumour activity in preclinical models of HER2-positive breast cancer (Munster et al, 2001; Munster et al, 2002). A phase-I study of tanespimycin plus trastuzumab was encouraging, and antitumour activity was observed in patients with HER2-positive MBC (Modi et al, 2007). In a subsequent single-arm phase-II trial of tanespimycin (IV weekly) plus trastuzumab in patients with HER2-positive MBC and disease progression following trastuzumab, an overall RR of 22% and CBR of 59% were reported (Modi et al, 2011). Tanespimycin was well-tolerated overall, with diarrhoea, fatigue, nausea and headache as the most common toxicities. Although further clinical development of tanespimycin has been halted, other hsp90 inhibitors, including retaspimycin (IPI-504; Infinity Pharmaceuticals, Cambridge, MA, USA) and AUY922 (Novartis, Cambridge, MA, USA) are currently under evaluation in early-phase clinical trials as single agents or in combination with trastuzumab (NCT00817362, NCT00526045 and NCT01271920).
PI3K/Akt/mTOR pathway modulation
Another strategy to combat trastuzumab resistance involves modulation of the PI3K/Akt/mTOR pathway (Nahta and O’Regan, 2010). Evaluation of the mTOR inhibitor everolimus (RAD001, Afinitor; Novartis) in HER2-positive breast cancer is the most advanced in clinical development to date. In a preclinical study of PTEN-deficient, trastuzumab-resistant in vitro and in vivo models, the combination of everolimus and trastuzumab resulted in enhanced antitumour effects (Lu et al, 2007). In phase-I trials, promising clinical activity was reported with everolimus when used in combination with paclitaxel/trastuzumab or vinorelbine/trastuzumab in HER2-positive MBC (Andre et al, 2010; Jerusalem et al, 2011). In the first trial, a phase-Ib dose-escalation study of everolimus with weekly paclitaxel and trastuzumab, an overall RR of 44% was reported among 27 evaluable patients (Andre et al, 2010). In all, 74% of patients experienced disease control for >6 months, with a median PFS of 34 weeks for the entire cohort. At the established dose of 10 mg per day, grade 3 and 4 neutropenia were the most common AEs (52%). In the second phase-Ib trial, which evaluated everolimus plus weekly vinorelbine and trastuzumab in HER2-positive MBC patients pretreated with trastuzumab, an overall RR of 19.1%, disease control rate of 83.0% and median PFS of 30.7 weeks were reported for the 47 evaluable patients (Jerusalem et al, 2011). Neutropenia (92%) and stomatitis (70%) were the most common hematologic and nonhematologic toxicities, respectively. Additionally, three patients developed febrile neutropenia and four patients received G-CSF support. Based on these promising early-phase clinical data in HER2-positive MBC, phase-III trials of trastuzumab/paclitaxel±everolimus in the first-line setting (BOLERO-1; NCT00876395) and trastuzumab/vinorelbine±everolimus in the trastuzumab-refractory setting (BOLERO-3; NCT01007942) are in progress. Early-phase clinical trials are currently evaluating modulation of the PI3K/Akt/mTOR pathway with the use of PI3K/Akt inhibitors, including BKM120 (Novartis) and BEZ-235 (Novartis) (NCT00620594 and NCT01132664). Preclinical studies with PI3K/Akt inhibitors suggest increased activity in tumours with PIK3CA mutations (Brachmann et al, 2009), identified in approximately 20 to 30% of HER2-positive breast cancers (Saal et al, 2005; Stemke-Hale et al, 2008; Gonzalez-Angulo et al, 2011).
IGF-1R inhibitors
Crosstalk between HER2 and IGF receptor families leading to activation of alternative signaling pathways has also been implicated in trastuzumab resistance (Nahta et al, 2006). Preclinical models of trastuzumab-resistant, HER2-positive breast cancer have characterised restoration of trastuzumab sensitivity by disrupting the IGF-1R/HER2 heterodimer, synergistic interactions with trastuzumab and associated decreased downstream receptor signaling with IGF-1R inhibition (Lu et al, 2001; Nahta et al, 2005; Esparis-Ogando et al, 2008). In phase-I trials of IGF-1R monoclonal antibodies in advanced solid malignancies, these agents appear to be well-tolerated overall, although toxicities of thrombocytopenia and hyperglycemia were observed (Weroha and Haluska, 2008). Currently, phase-I and -II studies of anti-IGF-1R therapies are underway in patients with locally advanced or metastatic HER2-positive breast cancer after trastuzumab failure, including a phase-I/II study of the small-molecule inhibitor BMS-754807 (Bristol-Myers Squibb) in combination with trastuzumab (NCT00788333) and a phase-II study of capecitabine/lapatinib with or without the monoclonal antibody cixutumumab (IMC-A12; ImClone, Bridgewater, NJ, USA) in patients previously treated with trastuzumab and an anthracycline and/or a taxane (NCT00684983).
Conclusions
There is no doubt that trastuzumab has provided significant clinical benefit in patients with HER2-positive breast cancer. Still, primary (de novo) and secondary (acquired) resistance represents a real clinical challenge. Lapatinib has demonstrated modest clinical activity in this setting and highlights the importance of ongoing HER2 blockade in trastuzumab-refractory states. Newer HER-family TKIs that are both irreversible and target HER4 in addition to EGFR and HER2 are being evaluated and may provide superior outcomes in this population. Meanwhile, anti-VEGF strategies, such as bevacizumab, have demonstrated promising activity and phase-III results are eagerly awaited. Other investigational agents in HER2-positive MBC, including hsp90 and mTOR inhibitors, utilise novel approaches to combat trastuzumab resistance and have also shown promising activity in early-phase clinical trials. IGF-1R inhibition is supported by biologic rationale in the setting of trastuzumab resistance due to receptor crosstalk, but mature clinical data are lacking. The two antibody-based HER2-directed approaches, pertuzumab and T-DM1, have shown promising efficacy. Both agents are currently under phase-III evaluation and have the potential to establish new treatment paradigms. Finally, ongoing translational research is critical in the development of novel biomarkers predictive of clinical benefit with these evolving targeted agents in HER2-positive breast cancer in order to minimise untoward drug-related toxicities and ultimately enhance patient outcomes.
References
Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX (2002) Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2: 127–137
Andre F, Campone M, O’Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA (2010) Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol 28: 5110–5115
Baselga J, Gelmon KA, Verma S, Wardley A, Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, Fumoleau P, Gianni L (2010) Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 28: 1138–1144
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van d, V, Bernards R (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12: 395–402
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2010) Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28: 1124–1130
Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, Forster JK, Rubin SD, Stein SH, Burstein HJ (2009) Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol 20: 1026–1031
Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM (2009) Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A 106: 22299–22304
Burris III HA, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O’Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, Harris JL, Smith DA, Koch KM, Stead A, Mangum S, Spector NL (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23: 5305–5313
Burris III HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA, Girish S, Amler L, Zheng M, Chu YW, Klencke B, O’Shaughnessy JA (2010) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29: 398–405
Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD (2008a) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26: 1810–1816
Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, Salazar VM, Blackwell KL (2008b) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19: 1068–1074
Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, Abbas R, Powell C, Turnbull K, Vermette J, Zacharchuk C, Badwe R (2010) Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28: 1301–1307
Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE (2010) Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 15: 924–934
Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, Chan A, Campone M, Viens P, Davidson N, Gorbounova V, Raats JI, Skarlos D, Newstat B, Roychowdhury D, Paoletti P, Oliva C, Rubin S, Stein S, Geyer CE (2008) A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 112: 533–543
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH, Williams LS, Dering J, Finn RS, Press MF (2008) Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 26: 5544–5552
Dickler M, Franco S, Stopeck A, Ma W, Nulsen B, Lyandres J, Melisko M, Lahiri S, Arbushites M, Koehler M, Rugo HS (2008) Final results from a phase II evaluation of lapatinib (L) and bevacizumab (B) in HER2-overexpressing metastatic breast cancer (MBC). Poster presented at: the 31st Annual CTRC-AACR San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX, USA. Abstract 3133
Dieras V, Harbeck N, Albain K, Burris H, Awada A, Crivellari D, Andre F, Choi YJ, Huang J, Miller KD (2010) A phase Ib/II trial of trastuzumab-DM1 with pertuzumab for patients with HER2-positive, locally advanced or metastatic breast cancer: interim efficacy and safety results. Presented at: 33rd Annual San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. Abstract P3-14-01
Dokmanovic M, Hirsch DS, Shen Y, Wu WJ (2009) Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther 8: 1557–1569
Esparis-Ogando A, Ocana A, Rodriguez-Barrueco R, Ferreira L, Borges J, Pandiella A (2008) Synergic antitumoral effect of an IGF-IR inhibitor and trastuzumab on HER2-overexpressing breast cancer cells. Ann Oncol 19: 1860–1869
Finn RS, Press M, Dering J, Florance A, Platek G, Arbushites M, Koehler M, Johnston S (2009a) Progression-free survival (PFS) of patients with HER2-negative estrogen-resepctor (ER)-low metastatic breast cancer (MBC) with the addition of lapatinib to letrozole: biomarker results of EGF30008. J Clin Oncol 27. Abstract 1018
Finn RS, Press MF, Dering J, Arbushites M, Koehler M, Oliva C, Williams LS, Di LA (2009b) Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol 27: 3908–3915
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5: 317–328
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733–2743
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, Burgues O, Lluch AM, Chen H, Hortobagyi GN, Mills GB, Meric-Bernstam F (2011) PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 10: 1093–1101
Hickish T, Wheatley D, Lin N, Carey LA, Houston S, Mendelson DS, Solca F, Uttenreuther-Fischer M, Winer E (2009) Use of BIBW 2992, a novel irreversible EGFR/HER1 and HER2 tyrosine kinase inhibitor to treat patients with HER2-positive metastatic breast cancer after failure of treatment with trastuzumab. Cancer Res 69: 2191–2194. Abstract 5060
Hurvitz S, Dirix L, Kocsis J, Gianni L, Lu J, Vinholes J, Song C, Tong B, Chu YW, Perez EA (2011) Trastuzumab emtansine (T-DM1) vs trastuzumab plus docetaxel (H+T) in previously-untreated HER2-positive metastatic breast cancer (MBC): primary results of a randomized, multicenter, open-label phase II study (TDM4450g/B021976). Eur J Cancer 47: S330. Abstract 5001
Hurvitz SA, Pegram MD, Lin L-S, Chan DS, Allen HJ, Dichmann RA, Hagenstad CT, Barstis J, Hermann RC, Hu EH, Moroose RL, Thomas SP, Vogel CL, Ryba N, Elashoff D, Slamon DJ (2009) Final results of a phase II trial evaluating trastuzumab and bevacizumab as first line treatment of HER2-amplified advanced breast cancer. Presented at: Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. Abstract 6094
Jerusalem G, Fasolo A, Dieras V, Cardoso F, Bergh J, Vittori L, Zhang Y, Massacesi C, Sahmoud T, Gianni L (2011) Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat 125: 447–455
Johnston S, Pippen Jr J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27: 5538–5546
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX (2010) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128: 347–356
Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, Beryt M, Hepp H, Slamon DJ, Pegram MD (2004) Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 10: 1706–1716
Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ (2006) Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66: 1630–1639
Krop I, Lo RP, Miller K, Modi S, Yardley DA, Rodriguez G, Agresta S, Zheng M, Amler L, Rugo H (2009a) A phase II study of trastuzumab-DM1 (T-DM1), a novel HER2 antibody-drug conjugate, in patients previously treated with lapatinib, trastuzumab, and chemotherapy. Cancer Res 69: 795s. Abstract 5090
Krop I, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G, Agresta S, Zheng M, Amler L, Rugo H (2009b) A phase II study of trastuzumab-DM1 (T-DM1), a novel HER2 antibody-drug conjugate, in patients with HER2+ metastatic breast cancer who were previously treated with an anthracycline, a taxane, capecitabine, lapatinib, and trastuzumab. Presented at: the Annual CTRC-AACR San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. Abstract 5090
Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, Girish S, Tibbitts J, Yi JH, Sliwkowski MX, Jacobson F, Lutzker SG, Burris HA (2010) Phase I study of trastuzumab-DM1, a HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28: 398–405
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68: 9280–9290
Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27: 4702–4711
Lin NU, Mayer IA, Najita JS, Hobday TJ, Falkson CI, Dees EC, Rimawi MF, Nanda R, Gelman RS, Josephs K, Richardson A, Flores L, Van den Abbeele AD, Yap JT, Arteaga CL, Wolff AC, Krop IE, Winer EP (2011) TBCRC 003: Phase II trial of trastuzumab (T) and lapatinib (L) in patients (pts) with HER2+ metastatic breast cancer (MBC). J Clin Oncol 29: Abstract 527
Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, Mills GB, Hortobagyi GN, Esteva FJ, Yu D (2007) Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res 13: 5883–5888
Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93: 1852–1857
Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, Cropp GF, Johnson RG, Hannah AL, Hudis CA (2007) Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol 25: 5410–5417
Modi S, Stopeck AT, Linden HM, Solit DB, Chandarlapaty S, Rosen N, D’Andrea G, Dickler MN, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C (2011) Hsp90 inhibition is effective in breast cancer: A phase 2 trial of tanespimycin (17AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 17: 5132–5139
Munster PN, Marchion DC, Basso AD, Rosen N (2002) Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res 62: 3132–3137
Munster PN, Srethapakdi M, Moasser MM, Rosen N (2001) Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res 61: 2945–2952
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127
Nahta R, Hung MC, Esteva FJ (2004) The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64: 2343–2346
Nahta R, O’Regan RM (2010) Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway. Clin Breast Cancer 10: S72–S78
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ (2006) Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3: 269–280
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65: 11118–11128
Perez EA, Dirix L, Kocsis J, Gianni L, Lu J, Vinholes J, Ng V, Linehan C, Agresta S, Hurvitz S (2010) Efficacy and safety of trastuzumab-DM1 vs trastuzumab plus docetaxel in HER2-positive metastatic breast cancer patients with no prior chemotherapy for metastatic disease: preliminary results of a randomized, multicenter, open-label phase 2 study (TDM4450G). Ann Oncol 21: viii2. Abstract LBA3
Portera CC, Walshe JM, Rosing DR, Denduluri N, Berman AW, Vatas U, Velarde M, Chow CK, Steinberg SM, Nguyen D, Yang SX, Swain SM (2008) Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res 14: 2710–2716
Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A (2004) Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64: 3958–3965
Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM (2001) The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 1: 85–94
Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg A, Parsons R (2005) PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65: 2554–2559
Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di CS, Matias-Guiu X, Cajal S, Arribas J, Baselga J (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99: 628–638
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69: 9330–9336
Slamon D, Gomez HL, Kabbinavar FF, Amit O, Richie M, Pandite L, Goodman V (2008) Randomized study of pazopanib + lapatinib vs lapatinib alone in patients with HER2-positive advanced or metastatic breast cancer. J Clin Oncol 26: 45s. Abstract 1016
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Spector NL, Blackwell KL (2009) Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 27: 5838–5847
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung MC, van d, V, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68: 6084–6091
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic Hsp90 complex in cancer. Nat Rev Cancer 10: 537–549
Weroha SJ, Haluska P (2008) IGF-1 receptor inhibitors in clinical trials--early lessons. J Mammary Gland Biol Neoplasia 13: 471–483
Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, Janne PA, Eder JP, Naughton MJ, Ellis MJ, Jones SF, Mekhail T, Zacharchuk C, Vermette J, Abbas R, Quinn S, Powell C, Burris HA (2009) A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 15: 2552–2558
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL (2002) Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and Akt pathways. Oncogene 21: 6255–6263
Yamamoto N, Katakami N, Atagi S, Hida T, Goto K, Horai T, Inoue A, Ichinose Y, Kobayashi K, Takeda K, Kiura K, Saka H, Tamura T, Okamoto I, Nogami N, Moringa R, Nishio K, Seki Y, Lorence R, Shahidi M (2011) A phase II trial of afatinib (BIBW 2992) in patients (pts) with advanced non-small cell lung cancer previously treated with erlotinib or gefitinib. Poster presented at: the Annual Meeting of the American Society of Clinical Oncology, June 4–8, 2011; Chicago, IL. Abstract 7524
Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D (2002) ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer 94: 2855–2861
Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, Ang J, Temple G, Bell S, Shahidi M, Uttenreuther-Fischer M, Stopfer P, Futreal A, Calvert H, de Bono JS, Plummer R (2010) Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 28: 3965–3972
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137
Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, Meloche S, aoui-Jamali MA (2000) Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene 19: 3460–3469
Acknowledgements
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). Editorial assistance was provided by Lisa Shannon, PharmD, of MedErgy, which was contracted by BIPI for these services. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development. The authors received no compensation related to the development of the manuscript. The manuscript is the sole product of the authors and no writing assistance was obtained. RYT was a Research Fellow of the Terry Fox Foundation (award #020017), Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tsang, R., Finn, R. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br J Cancer 106, 6–13 (2012). https://doi.org/10.1038/bjc.2011.516
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.516
Keywords
This article is cited by
-
Immunotherapy for breast cancer: past, present, and future
Cancer and Metastasis Reviews (2016)
-
The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling
Oncogene (2015)
-
Targeting HER2 Positive Breast Cancer with Chemopreventive Agents
Current Pharmacology Reports (2015)
-
Trastuzumab in advanced breast cancer – a decade of experience in Germany
BMC Cancer (2014)
-
MTDH mediates trastuzumab resistance in HER2 positive breast cancer by decreasing PTEN expression through an NFκB-dependent pathway
BMC Cancer (2014)