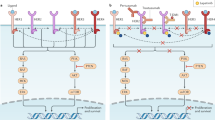
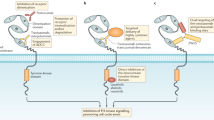
Abstract
Amplification and/or overexpression of ERBB2, the gene encoding HER2, can be found in 15–20% of invasive breast cancers and is associated with an aggressive phenotype and poor clinical outcomes. Relentless research efforts in molecular biology and drug development have led to the implementation of several HER2-targeted therapies, including monoclonal antibodies, tyrosine-kinase inhibitors and antibody–drug conjugates, constituting one of the best examples of bench-to-bedside translation in oncology. Each individual drug class has improved patient outcomes and, importantly, the combinatorial and sequential use of different HER2-targeted therapies has increased cure rates in the early stage disease setting and substantially prolonged survival for patients with advanced-stage disease. In this Review, we describe key steps in the development of the modern paradigm for the treatment of HER2-positive advanced-stage breast cancer, including selecting and sequencing new-generation HER2-targeted therapies, and summarize efficacy and safety outcomes from pivotal studies. We then outline the factors that are currently known to be related to resistance to HER2-targeted therapies, such as HER2 intratumoural heterogeneity, activation of alternative signalling pathways and immune escape mechanisms, as well as potential strategies that might be used in the future to overcome this resistance and further improve patient outcomes.
Key points
-
The discovery of ERBB2 and the clinical characterization of HER2, the protein it encodes, as a biomarker and therapeutic target have been key milestones in breast cancer treatment.
-
The development of several HER2-targeted agents, including monoclonal antibodies, tyrosine-kinase inhibitors and antibody–drug conjugates, has increased cure rates in patients with early stage HER2-positive (HER2+) breast cancer and substantially improved survival for patients with advanced-stage HER2+ breast cancer.
-
Incorporating novel HER2-targeted therapies into treatment algorithms for patients with metastatic HER2+ breast cancer to optimize selection and sequencing of these agents is currently a clinical challenge.
-
Several mechanisms, such as intratumoural heterogeneity, activation of alternative signalling pathways and immune escape mechanisms, can reduce the efficacy of HER2-targeted agents.
-
Future research on treatments for patients with HER2+ breast cancer is focusing on developing novel drugs that can specifically target cancer cells with minimal side effects as well as on the implementation of novel technologies, such as artificial intelligence for integrating clinical and genomic data, liquid biopsy assays for minimal residual disease monitoring and treatment escalation/de-escalation, and single-cell sequencing for a more precise assessment of HER2 heterogeneity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carpenter, G., King, L. Jr & Cohen, S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature 276, 409–410 (1978).
Schechter, A. L. et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312, 513–516 (1984).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Schlam, I. & Swain, S. M. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer 7, 56 (2021).
Coussens, L. et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230, 1132–1139 (1985).
Kraus, M. H., Issing, W., Miki, T., Popescu, N. C. & Aaronson, S. A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl Acad. Sci. USA 86, 9193–9197 (1989).
Plowman, G. D. et al. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl Acad. Sci. USA 90, 1746–1750 (1993).
Di Fiore, P. P. et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 237, 178–182 (1987).
Hudziak, R. M., Schlessinger, J. & Ullrich, A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl Acad. Sci. USA 84, 7159–7163 (1987).
Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 (1989).
Drebin, J. A., Stern, D. F., Link, V. C., Weinberg, R. A. & Greene, M. I. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature 312, 545–548 (1984).
Drebin, J. A., Link, V. C., Weinberg, R. A. & Greene, M. I. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc. Natl Acad. Sci. USA 83, 9129–9133 (1986).
Drebin, J. A., Link, V. C., Stern, D. F., Weinberg, R. A. & Greene, M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 41, 697–706 (1985).
Fendly, B. M. et al. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 50, 1550–1558 (1990).
Carter, P. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Hudis, C. A. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Petricevic, B. et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 11, 307 (2013).
Collins, D. M. et al. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann. Oncol. 23, 1788–1795 (2012).
Varchetta, S. et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 67, 11991–11999 (2007).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
Klapper, L. N., Waterman, H., Sela, M. & Yarden, Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 60, 3384–3388 (2000).
Baselga, J. et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14, 737–744 (1996).
Pegram, M. D. et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 16, 2659–2671 (1998).
Cobleigh, M. A. et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 17, 2639–2648 (1999).
Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 12, S49–S55 (2001).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 27, 619–626 (2018).
Sundquist, M., Brudin, L. & Tejler, G. Improved survival in metastatic breast cancer 1985-2016. Breast 31, 46–50 (2017).
Grinda, T. et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open. 6, 100114 (2021).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119 (2012).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Junttila, T. T. et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 15, 429–440 (2009).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372, 724–734 (2015).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21, 519–530 (2020).
Bachelot, T. et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann. Oncol. 30, 766–773 (2019).
Miles, D. et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann. Oncol. 32, 1245–1255 (2021).
Rimawi, M. et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J. Clin. Oncol. 36, 2826–2835 (2018).
Johnston, S. R. D. et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: updated results of ALTERNATIVE. J. Clin. Oncol. 39, 79–89 (2021).
Arpino, G. et al. Pertuzumab, trastuzumab, and an aromatase inhibitor for HER2-positive and hormone receptor-positive metastatic/locally advanced breast cancer: PERTAIN final analysis. Clin. Cancer Res. 14, 1468–1476 (2023).
Hua, X. et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin. Cancer Res. 28, 637–645 (2022).
Perez, E. A. et al. Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET Cohort 1 final results. Breast Cancer Res. 18, 126 (2016).
Andersson, M. et al. Efficacy and safety of pertuzumab and trastuzumab administered in a single infusion bag, followed by vinorelbine: VELVET cohort 2 final results. Oncologist 22, 1160–1168 (2017).
Wildiers, H. et al. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): an open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group. Lancet Oncol. 19, 323–336 (2018).
Bazan, J. G., Holbrook, A., Jhawar, S. & White, J. R. Abstract P4-12-03: patterns of progression in metastatic breast cancer: does oligoprogression exist? Cancer Res. 80, P4-12-03–P14-12-03 (2020).
Lievens, Y. et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother. Oncol. 148, 157–166 (2020).
Patel, P. H., Palma, D., McDonald, F. & Tree, A. C. The dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin. Oncol. 31, 824–833 (2019).
Tsai, C. J. et al. Final analysis of consolidative use of radiotherapy to block (CURB) oligoprogression trial - a randomized study of stereotactic body radiotherapy for oligoprogressive metastatic lung and breast cancers. Int. J. Radiat. Oncol. Biol. Phys. 114, 1061 (2022).
Perez, E. A. et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J. Clin. Oncol. 35, 141–148 (2017).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
Blackwell, K. L. et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 28, 1124–1130 (2010).
Yamamoto, Y. et al. Pertuzumab retreatment for HER2-positive advanced breast cancer: a randomized, open-label phase III study (PRECIOUS). Cancer Sci. 113, 3169–3179 (2022).
Cameron, D. et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 15, 924–934 (2010).
von Minckwitz, G. et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J. Clin. Oncol. 27, 1999–2006 (2009).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Dieras, V. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 18, 732–742 (2017).
Marchio, C. et al. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 72, 123–135 (2021).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Saura Manich, C. et al. 279P Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol. 32, S485–S486 (2021).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J. Clin. Oncol. 38, 1887–1896 (2020).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20, 816–826 (2019).
Cortes, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117 (2023).
Andre, F. et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 401, 1773–1785 (2023).
Powell, C. A. et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 7, 100554 (2022).
Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106, 102378 (2022).
Tarantino, P. et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 7, 1873–1881, (2021).
Conte, P. et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 7, 100404 (2022).
Rugo, H. S., Bianchini, G., Cortes, J., Henning, J. W. & Untch, M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 7, 100553 (2022).
Huang, L., Jiang, S. & Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001-2020). J. Hematol. Oncol. 13, 143 (2020).
Moulder, S. L. et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC). Clin. Cancer Res. 23, 3529–3536 (2017).
Metzger Filho, O. et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann. Oncol. 31, 1231–1239 (2020).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Curigliano, G. et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann. Oncol. 33, 321–329 (2022).
Lin, N. U. et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 38, 2610–2619 (2020).
Lin, N. U. et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 9, 197–205, (2023).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Frenel, J.-S. et al. Efficacy of tucatinib+trastuzumab+capecitabine (TTC) after trastuzumab-deruxtecan (T-DXd) exposure in Her2-positive metastatic breast cancer: a French multicentre retrospective study. J. Clin. Oncol. 41, 1014–1014 (2023).
Saura, C. et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 32, 3626–3633 (2014).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Awada, A. et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2, 1557–1564, (2016).
Saura, C. et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J. Clin. Oncol. 38, 3138–3149 (2020).
Hurvitz, S. A. et al. Efficacy of neratinib plus capecitabine in the subgroup of patients with central nervous system involvement from the NALA trial. Oncologist 26, e1327–e1338 (2021).
Barcenas, C. H. et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann. Oncol. 31, 1223–1230 (2020).
Xu, B. et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22, 351–360 (2021).
Xu, B. et al. Abstract GS3-02: updated overall survival (OS) results from the phase 3 PHOEBE trial of pyrotinib versus lapatinib in combination with capecitabine in patients with HER2-positive metastatic breast cancer. Cancer Res. 82, GS3-02–GS03-02 (2022).
Ma, F. et al. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): randomised, double blind, multicentre, phase 3 trial. BMJ 383, e076065 (2023).
van der Lee, M. M. et al. The preclinical profile of the duocarmycin-based her2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 14, 692–703 (2015).
Saura Manich, C. et al. LBA15 primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 32, S1288 (2021).
Nordstrom, J. L. et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 13, R123 (2011).
Bang, Y. J. et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 28, 855–861 (2017).
Rugo, H. S. et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 7, 573–584, (2021).
Rugo, H. S. et al. Margetuximab versus trastuzumab in patients with previously treated HER2-positive advanced breast cancer (SOPHIA): final overall survival results from a randomized phase 3 trial. J. Clin. Oncol. 41, 198–205 (2023).
Malmgren, J., Hurlbert, M., Atwood, M. & Kaplan, H. G. Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990-2011. Breast Cancer Res. Treat. 174, 505–514 (2019).
Gallicchio, L., Devasia, T. P., Tonorezos, E., Mollica, M. A. & Mariotto, A. Estimation of the number of individuals living with metastatic cancer in the United States. J. Natl Cancer Inst. 114, 1476–1483 (2022).
Konecny, G. et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J. Natl Cancer Inst. 95, 142–153 (2003).
Dowsett, M. et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J. Clin. Oncol. 26, 1059–1065 (2008).
Gutierrez, M. C. et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J. Clin. Oncol. 23, 2469–2476 (2005).
Xia, W. et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc. Natl Acad. Sci. USA 103, 7795–7800 (2006).
Wang, Y. C. et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers-role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 13, R121 (2011).
Marra, A. & Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 5, 27 (2019).
Miller, T. W. et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 1, 338–351 (2011).
Landis, M. W., Pawlyk, B. S., Li, T., Sicinski, P. & Hinds, P. W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9, 13–22 (2006).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11, R77 (2009).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016).
Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin. Cancer Res. 26, 5820–5829 (2020).
Tolaney, S. M. et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 21, 763–775 (2020).
Tolaney, S. M. et al. Overall survival and exploratory biomarker analyses of abemaciclib plus trastuzumab with or without fulvestrant vs trastuzumab plus chemotherapy in HR+, HER2+ metastatic breast cancer patients. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-23-1209 (2023).
Vance, G. H. et al. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch. Pathol. Lab. Med. 133, 611–612 (2009).
Bernasconi, B., Chiaravalli, A. M., Finzi, G., Milani, K. & Tibiletti, M. G. Genetic heterogeneity in HER2 testing may influence therapy eligibility. Breast Cancer Res. Treat. 133, 161–168 (2012).
Bartlett, J. M. et al. HER2 testing in the UK: recommendations for breast and gastric in-situ hybridisation methods. J. Clin. Pathol. 64, 649–653 (2011).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Hanna, W. M. et al. HER2 in situ hybridization in breast cancer: clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 27, 4–18 (2014).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Perez, E. A. et al. Relationship between tumor biomarkers and efficacy in MARIANNE, a phase III study of trastuzumab emtansine ± pertuzumab versus trastuzumab plus taxane in HER2-positive advanced breast cancer. BMC Cancer 19, 517 (2019).
Hou, Y. et al. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res. Treat. 166, 447–457 (2017).
Rye, I. H. et al. Intratumor heterogeneity defines treatment-resistant HER2+ breast tumors. Mol. Oncol. 12, 1838–1855 (2018).
Hurvitz, S. A. et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J. Clin. Oncol. 37, 2206–2216 (2019).
Caswell-Jin, J. L. et al. Clonal replacement and heterogeneity in breast tumors treated with neoadjuvant HER2-targeted therapy. Nat. Commun. 10, 657 (2019).
Filho, O. M. et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 11, 2474–2487 (2021).
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18, 327–344 (2021).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Mosele, F. et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat. Med. 29, 2110–2120 (2023).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
She, Q. B. et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE 3, e3065 (2008).
Berns, K. et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12, 395–402 (2007).
Esteva, F. J. et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am. J. Pathol. 177, 1647–1656 (2010).
Chandarlapaty, S. et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 18, 6784–6791 (2012).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e426 (2018).
Loibl, S. et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann. Oncol. 27, 1519–1525 (2016).
Baselga, J. et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 32, 3753–3761 (2014).
Baselga, J. et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin. Cancer Res. 22, 3755–3763 (2016).
Andre, F. et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 15, 580–591 (2014).
Andre, F. et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J. Clin. Oncol. 34, 2115–2124 (2016).
Jhaveri, K. et al. A phase I study of alpelisib in combination with trastuzumab and LJM716 in patients with PIK3CA-mutated HER2-positive metastatic breast cancer. Clin. Cancer Res. 27, 3867–3875 (2021).
Solit, D. B. et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 (2006).
Smith, A. E. et al. HER2+ breast cancers evade anti-HER2 therapy via a switch in driver pathway. Nat. Commun. 12, 6667 (2021).
Ferraro, E. et al. Abstract P4-02-01: efficacy of HER2 ADCs against HER2 inhibitor resistance alterations in the PI3K and MAPK pathways in HER2-positive breast cancer. Cancer Res. 83, P4-02-01–P04-02-01 (2023).
Kancha, R. K. et al. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS ONE 6, e26760 (2011).
Xu, X. et al. HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2+ breast cancer. Clin. Cancer Res. 23, 5123–5134 (2017).
Kingston, B. et al. Genomic profile of advanced breast cancer in circulating tumour DNA. Nat. Commun. 12, 2423 (2021).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Holbro, T. et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl Acad. Sci. USA 100, 8933–8938 (2003).
Krishnamurthy, A. & Jimeno, A. Bispecific antibodies for cancer therapy: a review. Pharmacol. Ther. 185, 122–134 (2018).
Geuijen, C. A. W. et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell 33, 922–936.e910 (2018).
Hamilton, E. P. et al. Clinical activity of MCLA-128 (zenocutuzumab), trastuzumab, and vinorelbine in HER2 amplified metastatic breast cancer (MBC) patients (pts) who had progressed on anti-HER2 ADCs. J. Clin. Oncol. 38, 3093–3093 (2020).
Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug. Discov. 22, 101–126 (2023).
Krop, I. E. et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC). J. Clin. Oncol. 40, 1002–1002 (2022).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13, 228–241 (2016).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50 (2018).
Luen, S. J. et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 18, 52–62 (2017).
Hills, R. K. et al. Do tumor infiltrating lymphocytes (TILs) predict benefits from trastuzumab therapy for HER2 positive breast cancer? Meta-analysis of individual patient data from 4097 women in 5 trials. J. Clin. Oncol. 41, 508 (2023).
Datta, J. et al. Progressive loss of anti-HER2 CD4(+) T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. Oncoimmunology 4, e1022301 (2015).
Dirix, L. Y. et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 167, 671–686 (2018).
Chia, S. et al. A phase Ib trial of durvalumab in combination with trastuzumab in HER2-positive metastatic breast cancer (CCTG IND.229). Oncologist 24, 1439–1445 (2019).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20, 371–382 (2019).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21, 1283–1295 (2020).
Adams, S. et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404 (2019).
Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387, 217–226 (2022).
Geyer, J. C. E. et al. Abstract OT2-16-05: safety analyses of NRG BR004: a randomized, double-blind, phase III trial of taxane/trastuzumab/pertuzumab with atezolizumab or placebo in first-line HER2-positive metastatic breast cancer (MBC). Cancer Res. 83, OT2-16-05–OT12-16-05 (2023).
Gil Del Alcazar, C. R., Aleckovic, M. & Polyak, K. Immune escape during breast tumor progression. Cancer Immunol. Res. 8, 422–427 (2020).
Gil Del Alcazar, C. R. et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 7, 1098–1115 (2017).
Linde, N. et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 9, 21 (2018).
Shi, Y. et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcgamma receptors on macrophages. J. Immunol. 194, 4379–4386 (2015).
Betancur, P. A. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 8, 14802 (2017).
Upton, R. et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc. Natl Acad. Sci. USA 118, e2026849118 (2021).
Messaoudene, M. et al. T-cell bispecific antibodies in node-positive breast cancer: novel therapeutic avenue for MHC class I loss variants. Ann. Oncol. 30, 934–944 (2019).
Blanco, B., Dominguez-Alonso, C. & Alvarez-Vallina, L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin. Cancer Res. 27, 5457–5464 (2021).
van Hall, T. et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer 7, 263 (2019).
Geurts, V. C. M. et al. Unleashing NK- and CD8 T cells by combining monalizumab and trastuzumab for metastatic HER2-positive breast cancer: results of the MIMOSA trial. Breast 70, 76–81 (2023).
Echle, A. et al. Deep learning in cancer pathology: a new generation of clinical biomarkers. Br. J. Cancer 124, 686–696 (2021).
Boehm, K. M., Khosravi, P., Vanguri, R., Gao, J. & Shah, S. P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer 22, 114–126 (2022).
Moding, E. J., Nabet, B. Y., Alizadeh, A. A. & Diehn, M. Detecting liquid remnants of solid tumors: circulating tumor DNA minimal residual disease. Cancer Discov. 11, 2968–2986 (2021).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 15, 689–699 (2014).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 18, 743–754 (2017).
Perez, E. A. et al. Incidence of adverse events with therapies targeting HER2-positive metastatic breast cancer: a literature review. Breast Cancer Res. Treat. 194, 1–11 (2022).
Battisti, N. M. L., Tong, D., Ring, A. & Smith, I. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: the Royal Marsden experience. Breast Cancer Res. Treat. 178, 401–408 (2019).
Yeo, B., Kotsori, K., Mohammed, K., Walsh, G. & Smith, I. E. Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast 24, 751–757 (2015).
Tripathy, D. et al. De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist 25, e214–e222 (2020).
Wong, Y. et al. Long-term survival of de novo stage IV human epidermal growth receptor 2 (HER2) positive breast cancers treated with HER2-targeted therapy. Oncologist 24, 313–318 (2019).
den Brok, W. D. et al. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res. Treat. 161, 549–556 (2017).
Harano, K. et al. Clinicopathological and surgical factors associated with long-term survival in patients with HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 159, 367–374 (2016).
Hopkins, A. M., Rowland, A., McKinnon, R. A. & Sorich, M. J. Predictors of long-term disease control and survival for HER2-positive advanced breast cancer patients treated with pertuzumab, trastuzumab, and docetaxel. Front. Oncol. 9, 789 (2019).
Yardley, D. A. et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br. J. Cancer 110, 2756–2764 (2014).
Witzel, I. et al. Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer - results from the HER-OS patient registry. BMC Cancer 14, 806 (2014).
Veitch, Z. et al. No evidence of disease versus residual disease in long-term responders to first-line HER2-targeted therapy for metastatic breast cancer. Br. J. Cancer 126, 881–888 (2022).
Kim, S. B. et al. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. treatment of physician’s choice in previously treated HER2-positive advanced breast cancer. Int. J. Cancer 139, 2336–2342 (2016).
Burris, H. A. 3rd et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2011).
Braso-Maristany, F. et al. HER2DX ERBB2 mRNA expression in advanced HER2-positive breast cancer treated with T-DM1. J. Natl Cancer Inst. 115, 332–336 (2023).
Garcia-Murillas, I. et al. Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol. 5, 1473–1478 (2019).
Ponde, N. F., Lambertini, M. & de Azambuja, E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 1, e000073 (2016).
Giordano, S. H. et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol. 40, 2612–2635 (2022).
Pantel, K. & Alix-Panabieres, C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 (2019).
Sammons, S., Van Swearingen, A. E. D., Chung, C. & Anders, C. K. Advances in the management of breast cancer brain metastases. Neurooncol Adv. 3, v63–v74 (2021).
Martin, A. M. et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 3, 1069–1077 (2017).
Brufsky, A. M. et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin. Cancer Res. 17, 4834–4843 (2011).
Pestalozzi, B. C. et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 14, 244–248 (2013).
Olson, E. M. et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22, 525–531 (2013).
Lin, N. U. et al. Abstract P2-13-05: central nervous system metastases as a site of first recurrence in adjuvant therapy trials of HER2+ early breast cancer (EBC). Cancer Res. 82, P2-13-05–P12-13-05 (2022).
Swain, S. M. et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann. Oncol. 25, 1116–1121 (2014).
Krop, I. E. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann. Oncol. 26, 113–119 (2015).
Duchnowska, R., Loibl, S. & Jassem, J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 67, 71–77 (2018).
Nader-Marta, G. et al. Efficacy of tyrosine kinase inhibitors for the treatment of patients with HER2-positive breast cancer with brain metastases: a systematic review and meta-analysis. ESMO Open. 7, 100501 (2022).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14, 64–71 (2013).
Freedman, R. A. et al. Translational breast cancer research consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 34, 945–952 (2016).
Freedman, R. et al. Abstract PD7-03: translational breast cancer research consortium trial 022: neratinib and trastuzumab-emtansine for HER2+ breast cancer brain metastases (BCBM). Cancer Res. 83, PD7-03–PD07-03 (2023).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Lin, N. U. et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J. Clin. Oncol. 39, 2667–2675 (2021).
Hurvitz, S. et al. Abstract GS3-01: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res. 82, GS3-01–GS03-01 (2022).
Bartsch, R. et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat. Med. 28, 1840–1847 (2022).
Perez-Garcia, J. M. et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol. 25, 157–166 (2023).
Kabraji, S. et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin. Cancer Res. 29, 174–182 (2023).
Acknowledgements
All authors acknowledge support from the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute (grant no P30CA008748). A.M. is supported by the European Society for Medical Oncology José Baselga Fellowship for Clinician Scientists founded by AstraZeneca (2023–2025). S.C. is supported by the Breast Cancer Research Foundation. The authors thank and appreciate the helpful comments and suggestions provided by the journal editors and reviewers.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to each stage of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M. has received honoraria as a consultant, adviser or speaker from Roche and Menarini/Stemline. S.C. receives institutional grants and/or funding from AstraZeneca, Daiichi Sankyo and Lilly; has shares and/or ownership interests in Odyssey Biosciences and Totus Medicines; and has received honoraria as a consultant or adviser from AstraZeneca, Lilly, Prelude Therapeutics, Neogenomics, Novartis, Nuvalent and SAGA Diagnostics Effector Therapeutics. S.M. receives institutional funding for clinical research from AstraZeneca, Daiichi Sankyo, Genentech and Seagen; and has received honoraria as a consultant, adviser or speaker from AstraZeneca, Daiichi Sankyo, Eli Lilly, Genentech, Gilead, GlaxoSmithKline, Macrogenics, Puma Biotechnology, Seagen and Zymeworks.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks J. Cortes; H. Iwata, who co-reviewed with K. Nozawa; and P. Tarantino, who co-reviewed with O. Martínez-Sáez, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marra, A., Chandarlapaty, S. & Modi, S. Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives. Nat Rev Clin Oncol 21, 185–202 (2024). https://doi.org/10.1038/s41571-023-00849-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00849-9