Abstract
Aim:
To investigate which endothelin receptors mediated isoproterenol (ISO)-induced downregulation of FKBP12.6/12 in cardiomyocytes and study whether argirhein, a novel compound containing rhein and L-arginine that has anti-inflammatory activity, could reverse the downregulation of FKBP12.6/12 induced by ISO.
Methods:
Neonatal rat cardiomyocytes were incubated with ISO to downregulate FKBP12.6/12. Then the cells were treated with a selective ETA blocker (PD156707) and a ETB blocker (IRL1038), a dual ETA/ETB antagonist (CPU0213), and argirhein, respectively. FKBP12.6/12 expression was assayed by RT-PCR, Western blot, and immunocytochemistry.
Results:
The expression of FKBP12.6 mRNA was reduced by 37.7% (P<0.01) and 28.9% (P<0.05) relative to the control by ISO 1 and 0.1 μmol/L, respectively, but no response to ISO 0.01 μmol/L was observed in vitro. FKBP12.6/12 protein expression was reduced by 47.2% (P<0.01) and 37.8% (P<0.05) by ISO 1 and 0.1 μmol/L, respectively. This decrease was reversed significantly by PD156707, or IRL1038, and CPU0213. CPU0213 was more potent than either PD156707 or IRL-1038. Argirhein 10 μmol/L blunted the downregulation of FKBP12.6/12 by ISO, as demonstrated by the rising mRNA and protein levels and by the fluorescent density of the ISO-incubated cardiomyocytes.
Conclusion:
In cardiomyocytes, the ISO induced downregulation of FKBP12.6/12 is modulated by both ETA and ETB. A new compound, argirein, reversed the down-regulation of FKBP12.6/12 expression in myocardial cells stimulated with ISO.
Similar content being viewed by others
Introduction
Severe arrhythmias and congestive heart failure have become major problems affecting humans, and the annual incidence of sudden cardiac death (SCD) in the general population is estimated to be 1 in 10001. Mutations in the potassium channels KCNQ2 and KCNH2, and in the sodium channel SCN5A serve as markers only for polymorphism, and severe arrhythmias may occur in the presence of triggering factors2. Trigger factors, including stress caused by β-adrenergic stimulation and some medications, affect K+ currents during repolarization, resulting in tachyarrhythmias in patients with mutated genes. Tricyclic antidepressants may trigger the appearance of Brugada syndrome (Brugada ECG and life-threatening ventricular arrhythmias) in patients with the SCN5A polymorphism (His558Arg)3. Mutation of the ryanodine receptor type 2 (RyR2) alone does not appear to cause tachyarrhythmias; however, patients with RyR2 mutations may present with CPVT (catecholaminergic polymorphism ventricular tachyarrhythmias) while engaging in physical exercise, which is always associated with β-adrenoceptor stimulation4. Life threatening arrhythmias are likely to occur in patients with cardiac disease such as heart failure, in which multiple steps are needed to trigger events, including profound activation of β-adrenoceptor5, 6, and many causal factors are implicated in the pathogenesis of failing hearts in which an excess of reactive oxygen species (ROS) and inflammatory factors exist, resulting from the downstream events of β-adrenergic stimulation. Some inflammatory factors that have been revealed in the pathogenesis of congestive heart failure may participate in mechanisms underlying life threatening arrhythmias in failing hearts7.
FKBP12.6 (calstabin 2) is a key subunit that binds to RyR2 at the sarcoplasmic reticulum (SR) and is involved in calcium handling activity in the myocardium. FKBP12.6 also plays a role in the molecular mechanisms underlying severe arrhythmias and cardiac insufficiency8. CPVT may be the consequence of dissociated/downregulated FKBP12.6/12 related to profound β-adrenoceptor activation that keeps RyR2 channels closed loosely, allowing calcium leaks during diastole. RyR2 is phosphorylated by protein kinase A (PKA)9. FKBP12.6/12 dissociation from the binding site at RyR2 is likely due to a decrease in affinity, but this hypothesis is still controversial10. Elevated free calcium levels during diastole contribute to an increased risk for both cardiac tachyarrhythmias and exacerbated cardiac failure. Downregulation of FKBP12.6/12 in cardiomyocytes can be reproduced by isoproterenol (ISO) and can be induced by either endothelin-1 (ET) or H2O211. The resulting calcium leaks can be predicted by the downregulation of FKBP12.6/1212; therefore, downregulated FKBP12.6/12 may be taken as a surrogate for calcium leaks, indicating an increased risk for severe arrhythmias and the deterioration of cardiac performance13.
ET-1 is a cytokine that actively participates in inflammatory reactions and ROS genesis through activating NADPH oxidase14. An activation of the ET system is always associated with NADPH oxidase, forming the ET-NADPH oxidase pathway that is implicated in many cardiovascular diseases15. The biological activities of ET-1 are the result of the stimulation of ET receptors A (ETA) and B (ETB). In our previous study, ET-1 was shown to cause the downregulation/dissociation of FKBP12.6/12 in cardiomyocytes11; however, it is unclear whether ETA and ETB play separate roles in this process.
Argirhein, a new synthetic compound, contains rhein linked to L-arginine with a hydrogen bond in its moiety (Figure 1). Rhein relieves liver fibrosis and injury through anti-inflammatory activity16, 17. When argirhein is used as a medication, rhein can be released from argirhein and then display its anti-inflammatory activity, which may protect cardiomyocytes from ISO-induced insults; therefore, the activity of argirhein could be relevant in attenuating the downregulation of FKBP12.6/12 at the SR. We hypothesized that the downregulation of FKBP12.6/12 by ISO causes a disturbance in calcium homeostasis, which worsens arrhythmogenesis and cardiac performance. This downregulation could be mediated by ETA and ETB individually. Given the anti-inflammatory activity of rhein, argirhein may have an activity similar to that of ET blockers, which play a role in protecting the myocardium by alleviating ISO-induced downregulation of FKBP12.6/12.
Materials and methods
Animals
Animal handling procedures were conducted in accordance with the Laboratory Animal Regulations of the Bureau of Science and Technology, Jiangsu Province, China.
Reagents
Isoproterenol was purchased from Shanghai Hefeng Medicine Company (Shanghai, China). PD156707, a selective endothelin receptor A antagonist, and IRL-1038, a selective endothelin B receptor antagonist, were purchased from Sigma and Genscript Corporation, USA, respectively. M-MLV (Promega, USA) and Taq DNA Polymerase (Tiangne) were purchased from Nanjing Tianwei Corp, China. Polyclonal goat anti-FKBP12.6/12 IgG was obtained from Santa Cruz Biotechnology Inc, USA. Polyclonal rabbit anti-actin-IgG and FITC-conjugated rabbit anti-goat IgG were purchased from Boster, Wuhan, China. HRP-conjugated rabbit anti-goat IgG was from Dako, USA. Argirhein (AR) was sourced from Zhejiang Chinese Medical University.
Cell culture
Neonatal Sprague-Dawley rats were obtained from the Experimental Animal Center of Nanjing. Neonatal ventricular myocytes were obtained and cultured as described previously9. Briefly, myocytes were obtained and cultured in 20% FBS-DMEM culture medium with BrdU to suppress the growth of fibroblasts. The culture was changed to serum-free DMEM medium after three days, at which time the myocytes reached confluence. Except for the control groups, myocytes were incubated with ISO (0.01, 0.1, or 1 μmol/L) for 18 h to determine the appropriate ISO concentration. Cells were incubated with PD156707, IRL-1038, CPU0213 and argirhein (AR) at the 3 doses to prevent the adverse effects of ISO on FKBP12.6/12 in cardiomyocytes.
Semi-quantitative RT-PCR
After an 18-h incubation, RNA was extracted with Trizol solution, and cDNA was synthesized as described previously12. RT-PCR was performed in a volume of 25 μL, and the products were detected in 2% agarose. The target gene was quantified using β-actin as an internal control. The sequences of the forward and reverse primers for FKBP12.6 (length 427 bp) and β-actin (580 bp) are listed below: 5′-AAGGAAGGACGGAAGTG-3′ and 5′-GAATAGAAGACAACCGACG-3′; and 5′-GCCTCAAGATCATCAGCAAT-3′ and 5′-AGGTCCACCACTGACACCTT-3′, respectively. The densities of the bands were analyzed using a gel imaging analysis system (GeneGenius, Syngene, England), and the relative density of each DNA band was obtained by dividing by the density of β-actin.
Western blotting
Protein was extracted from the incubated myocytes as described previously18. Briefly, after determination of the protein concentration, the supernatant was stored at −20 °C before use. Aliquots of samples were heated to 98 °C in a loading buffer and fractionated using 10% SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane that was blocked with nonfat milk (5% w/v). The blocked nitrocellulose membranes were incubated at 4 °C overnight with the specific primary antibody. After 3 washes, the blots were incubated with horseradish peroxidase (HRP)-conjugated goat secondary antibody IgG for 1 h at room temperature. Antigen was detected with a DAB kit, visualized by imaging acquisition and quantified by densitometry. The relative abundance was obtained by normalizing the density of the FKBP12.6/12 protein against that of β-actin.
Immunocytochemical analysis
Cardiomyocytes were fixed with cold acetone for 10 min after incubation with ISO for 18 h. After the cells were dried at room temperature, the cell membrane permeability was increased by incubation with 1% Triton X-100 for 1 h. Then, cardiomyocytes were sealed with 2% bovine serum albumin for 1 h and incubated overnight with polyclonal goat anti-FKBP12.6/12-IgG. After being washed 3 times with PBS, the cardiomyocytes were incubated with FITC-conjugated rabbit anti-goat IgG for 1 h. Finally, cardiomyocytes were imaged by fluorescence microscopy and the gray density was assayed and compared19.
Statistical analysis
All data are presented as the mean±SD and were analyzed with SPSS version 11.5 (USA). For statistical evaluation, a one-way analysis of variance was used, following Dunnett's test. The Student Newman Keuls test was performed when the variances were equal, and the Games-Howell test was used when the variances were not equal. A probability value of P<0.05 was considered statistically significant.
Results
Downregulation of FKBP12.6/12
The downregulation of FKBP12.6/12 in cardiomyocytes responded to ISO at 3 doses in vitro. The expression of FKBP12.6 mRNA was reduced by 37.7% (P<0.01) and 28.9% (P<0.05) relative to the control at ISO concentrations of 1 μmol/L and 0.1 μmol/L, respectively, but no response to ISO 0.01 μmol/L was observed. A reduction in FKBP12.6/12 protein expression of the same magnitude was found by Western blotting; the reductions were 47.2% (P<0.01) and 37.8% (P<0.05) for 1 μmol/L and 0.1 μmol/L, respectively (Figure 2A, 2B). Based on these results, an ISO concentration of 1 μmol/L was adopted for further experiments.
Responses to ET antagonists
The expression levels of FKBP12.6/12 mRNA and protein were downregulated in the presence of ISO, and then selective blockade of either ETA or ETB was analyzed. The ETA antagonist PD156707 suppressed the changes in protein abundance significantly at concentrations of 0.1 and 1 μmol/L relative to ISO alone (Figure 3A, 3B). It was found that the ETB antagonist IRL-1038 at a concentration of 1 μmol/L only induced a reduction in the downregulation of FKBP12.6/12 toward the normal level (Figure 3D). At 0.1 μmol/L, PD156707 was able to upregulate the abundance of FKBP12.6/12 remarkably; however, IRL1038 had no significant effect. Thus, ETA was more potent than ETB in modulating changes in FKBP12.6/12 induced by ISO. mRNA and protein expression for FKBP12.6/12 were not changed in the absence of ISO after an 18-h incubation with either PD156707 or IRL-1038 alone (Figure 3E, 3F).
Selective ETA antagonist PD156707 (A, B) and ETB antagonist IRL1038 (C, D) reversed the ISO-induced downregulated mRNA and protein expression of FKBP12.6/12 normalized by β-actin in neonatal rat cardiomyocytes, but the expression of FKBP12.6/12 was not affected when incubation with drugs PD156707 and IRL1038 in the absence of ISO (E,F). n=6. Mean±SD. cP<0.01 vs control. eP<0.05, fP<0.01 vs ISO.
CPU0213, a dual ETA and ETB receptor antagonist, was added at concentrations ranging from 0.01 to 1 μmol/L to test its effects on the changes in FKBP12.6/12 expression induced by ISO. CPU0213 was able to significantly reverse the downregulation of the levels of mRNA and protein of FKBP12.6/12 at all three ISO concentrations. At a concentration as low as 0.01 μmol/L, CPU0213 was able to elevate the depressed FKBP12.6/12 levels significantly, while no response was observed to either PD156707 or IRL-1038. It appears that a combined blockade of the two subtypes of ET receptors is more potent than a single selective blockade (Figure 4).
Argirhein upregulates FKBP12.6/12
Compared with the ISO group, the argirhein group showed a significant reversal of the ISO-induced downregulation of FKBP12.6/12 when argirhein was used at 10 μmol/L (Figure 5A, 5B). The activity of argirhein was similar to those of the ET antagonists and was less effective than either CPU0213 or PD156707. Cardiomyocytes incubated with argirhein alone has not altered expression of FKBP12.6/12 mRNA and protein (Figure 5C). This result indicates that argirhein counteracts the adverse effects of ISO on FKBP12.6/12 in cardiomyocytes.
Immunocytochemistry of FKBP12.6/12
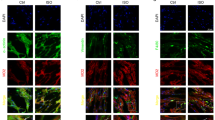
In an immunocytochemistry assay, the fluorescence intensity of FKBP12.6/12 in single incubated cardiomyocytes was bright in untreated cells but appeared to be faint after exposure to ISO for 18 h (Figure 6A, 6B, 6O), clearly indicating the downregulation/dissociation of FKBP12.6/12 by ISO in cardiomyocytes. Both ETA and ETB antagonists (PD156707 and IRL1038, respectively) enhanced the fluorescence intensity of cardiomyocytes in a dose-dependent manner (Figure 6C–6H, 6O). The effect of IRL1038 was impressive but less potent. Additionally, the novel compound argirhein was shown to increase the fluorescence intensity of FKBP12.6/12 at concentrations of 10−6 and 10−5 mol/L (Figure 6J, 6K, 6O). The dual endothelin receptor antagonist CPU0213 was potent in escalating the fluorescence in single cardiomyocytes and the mean gray value was higher than that of either PD156707 or IRL1038 (Figure 6L–6N, 6O). These results indicate that ISO-induced FKBP12.6/12 downregulation is modulated dramatically by either ETA or ETB and that argirhein significantly reversed the changes in FKBP12.6/12 expression induced by ISO, but argirhein was less effective than PD156707 and CPU0213.
Downregulation of fluorescence intensity of FKBP12.6/12 in neonatal rat cardiomyocytes was induced by isoproterenol and was blunted by selective ETA antagonist PD156707, ETB antagonist IRL1038, dual ET antagonist CPU0213 and argirhein. (A) Control; (B) ISO (1 μmol/L); (C) ISO+PD156707 (0.01 μmol/L); (D) ISO+PD156707 (0.1 μmol/L); (E) ISO+PD156707 (1 μmol/L); (F) ISO+IRL1038 (0.01 μmol/L); (G) ISO+IRL1038 (0.1 μmol/L); (H) ISO+IRL1038 (1 μmol/L); (I) ISO+AR (0.1 μmol/L); (J) ISO+AR (1 μmol/L); (K) ISO+AR (10 μmol/L); (L) ISO+CPU0213 (0.01 μmol/L); (M) ISO+CPU0213 (0.1 μmol/L); (N) ISO+CPU0213 (1 μmol/L); (O) The gray density of immunocytochemistry of FKBP12.6. n=6. Mean±SD. cP<0.01 vs control. eP <0.05, fP <0.01 vs ISO. gP<0.05 vs PD156707. kP<0.05, lP<0.01 vs IRL1038.
Discussion
Profound stimulation of β-receptors is commonly found in hearts that manifest worsening of arrhythmogenesis and declines in heart performance20, 21. It is believed that ISO worsens cardiac function and increases the risk of severe ventricular tachyarrhythmias. These activities are thought to be related to an impairment of the calcium handling protein FKBP12.6/12 at the SR. Calcium leaks during diastole result from the downregulation of FKBP12.6/12, which loses the ability to control the calcium releasing channels (RyR2). This leads to slow down repolarization followed by prolonged APD (action potential duration), and causes EAD (early after depolarization), which predisposes the heart to tachyarrhythmias12. A significant reversal of the deterioration of cardiac function and arrhythmogenic trends of affected hearts can be achieved through rescuing the depressed the FKBP12.6/12 level by blocking the activity of the ET receptors using CPU0213, a dual endothelin receptor antagonist12, 19, 22. In addition to ET antagonists, there are some compounds that specifically normalize abnormal RyR2 (and FKBP12.6/12) resulting from calcium and potassium channel blocking agents such as JTV51923 and CPU86017, which was developed at our laboratory24, 25.
The downregulation of FKBP12.6/12 induced by ISO is not a single event and is associated with an increase in other inflammatory factors, including ET, ROS, leptin, and an activated NADPH oxidase, in mediating the adverse effects of strong β-adrenoceptor stimulation in the heart18, 19, 25. In this regard, the role of ISO in activating β-receptors to downregulate FKBP12.6 is mediated by ET receptors and related pathways. In the present study we show that a selective antagonist of ETA and ETB rescue the downregulation of FKBP12.6/12 individually, in which ETB exerted an effect comparable to that of ETA. This phenomenon suggests that ETB is actively implicated in the hyperadrenergic state and triggers events that worsen cardiac performance and ventricular tachyarrhythmias. In cardiac fibroblasts incubated with ISO, ETB plays a minor role in the upregulation of Cx43, MMP-2, MMP-9, and NADPH oxidase relative to ETA18. We found that CPU0213 is more effective than PD156707 and IRL-1038 in modulating FKBP12.6/12, which is consistent with the findings in a previous study18. Our findings are supported by evidence that ETA and ETB are located on the sympathetic nerve ending in the myocardium and control release of norepinephrine individually26.
Rhein, an active component of Rheum officinale Baill, has anti-inflammatory activity and helps to relieve hepatic fibrosis16. With the activity against inflammatory factors in the kidney, rhein has been shown to be effective in treating diabetic nephropathy either as a single therapy27, 28 or in combination with benazepril29. The solubility of rhein is low, and chemical modification is encouraged to improve its chemical properties. Di-acetyl-rhein (diacerein, diacerhein) was produced by adding two acetyl groups to the moiety and was launched for treating osteoarthritis in Europe30. Rhein functions as the active metabolite of diacerein in treating osteoarthritis by suppressing osteoarthritic chondrocytes and osteoclastic differentiation/survival30. Diacerein suppresses proinflammatory cytokine expression in nonobese diabetic (NOD) mice31. The extracellular matrix activity is modulated by rhein, mediated by inhibiting the ERK and JNK-AP-1 pathways32.
Argirhein is a new compound containing two active molecules, rhein and L-arginine. The compound easily dissociates to form the two compounds in the body. As a consequence, the pharmacological effects of argirhein are relevant to the anti-inflammatory activity of rhein. On the other hand, an improvement in endothelial cells could be achieved by releasing L-arginine, which is beneficial to the recovery of dysfunctions of the vascular endothelium. A normal vascular endothelium is essential for cardiac function7. Activated ETA and ETB which participate in inflammatory reactions have been shown to be inhibited by argirhein.
An increase in ROS, ET and other cytokines is implicated in the events following the application of ISO33, and in patients with ventricular tachyarrhythmias, inflammatory factors are critically involved in the pathogenesis, which is triggered by β-receptor activation, of conditions such as hyperthyroidism in which the incidence of cardiac arrhythmias is common, in association with exaggerated stimulation of β-adrenoceptors34, 35. As compared to those happened in acquired heart diseases genetic mutation cover only a small portion of severe arrhythmias, such as arrhythmogenic cardiomyopathy36 and arrhythmias in patients with Brugada syndrome37, indicating that inflammatory factors are likely the major causal factors implicated in the affected myocardium responsible for arrhythmogenesis.
In conclusion, the downregulation of FKBP12.6/12, a calcium-modulating protein at the sarcoplasmic reticulum, is a key event involved in the worsening of heart dysfunction and in the arrhythmogenesis caused by stress related to β-receptor stimulation. We demonstrated that both ETA and ETB played individual roles in mediating the downregulation of FKBP12.6/12 caused by ISO application. ETB is definitely an active participant in this regard. We also showed for the first time that argirhein, a new compound containing rhein and L-arginine, shared the activity attenuating the ISO-induced downregulation of FKBP12.6/12 with ET blockers. Argirhein is potential drug for use in relieving stress related exacerbation of cardiac failure and arrhythmias by rescuing downregulation of FKBP12.6/12.
Author contribution
Guo-lin ZHANG conducted the project, processed the data and prepared the manuscript. De-zai DAI and Tao XI designed the project, discussed the mechanisms underlying the results and revised the manuscript. Xiao-dong CONG and Yun ZHANG investigated the new compound argirhein and discussed the data. Yin DAI supervised the experiment and reviewed the collected data.
References
Sen-Chowdhry S, McKenna WJ . Sudden cardiac death in the young: A strategy for prevention by targeted evaluation. Cardiology 2006; 105: 196–206.
Gollob MH . Genetic profiling as a marker for risk of sudden cardiac death. Curr Opin Cardiol 2006; 21: 42–6.
Chow BJ, Gollob M, Birnie D . Brugada syndrome precipitated by a tricyclic antidepressant. Heart 2005; 91: 651.
Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 2003; 113: 829–40.
Tomaselli GF, Zipes DP . What causes sudden death in heart failure? Circ Res 2004; 95: 754–63.
Rubart M, Zipes DP . Mechanisms of sudden cardiac death. J Clin Invest 2005; 115: 2305–15.
Blum A: Heart failure--new insights. Isr Med Assoc J 2009; 11: 105–11.
Yano M, Yamamoto T, Ikeda Y, Matsuzaki M . Mechanisms of disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med 2006; 3: 43–52.
Blayney LM, Jones JL, Griffiths J, Lai FA . A mechanism of ryanodine receptor modulation by FKBP12/12.6, protein kinase A, and K201. Cardiovasc Res 2010; 85: 68–78.
Jiang D, Jones PP, Davis DR, Gow R, Green MS, Birnie DH, et al. Characterization of a novel mutation in the cardiac ryanodine receptor that results in catecholaminergic polymorphic ventricular tachycardia. Channels (Austin) 2010; 4: 302–10.
Li N, Jia N, Dai DZ, Hu C, Dai Y . Role of endothelin in the effects of isoprenaline on potassium currents and calsequestrin 2 expression in the heart. Clin Exp Pharmacol Physiol 2010; 37: 557–63.
Zhang Y, Huang ZJ, Dai DZ, Feng Y, Na T, Tang XY, et al. Downregulated FKBP12.6 expression and upregulated endothelin signaling contribute to elevated diastolic calcium and arrhythmogenesis in rat cardiomyopathy produced by l-thyroxin. Int J Cardiol 2008; 130: 463–71.
Györke S, Carnes C . Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol Ther 2008; 119: 340–54.
Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS . Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 2005; 315: 1058–64.
Dong F, Zhang X, Ren J . Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension 2006; 47: 222–9.
Guo MZ, Li XS, Xu HR, Mei ZC, Shen W, Ye XF . Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats. Acta Pharmacol Sin 2002; 23: 739–44.
Malaguti C, Vilella CA, Vieira KP, Souza GH, Hyslop S, Zollner Rde L . Diacerhein downregulate proinflammatory cytokines expression and decrease the autoimmune diabetes frequency in nonobese diabetic (NOD) mice. Int Immunopharmacol 2008; 8: 782–91.
Peng HJ, Dai DZ, Ji H, Dai Y . The separate roles of endothelin receptors participate in remodeling of matrix metalloproteinase and connexin 43 of cardiac fibroblasts in maladaptive response to isoproterenol. Eur J Pharmacol 2010; 634: 101–6.
Feng Y, Tang XY, Dai DZ, Dai Y . Reversal of isoproterenol-induced downregulation of phospholamban and FKBP12.6 by CPU0213-mediated antagonism of endothelin receptors. Acta Pharmacol Sin 2007; 28: 1746–54.
Chelu MG, Wehrens XH . Sarcoplasmic reticulum calcium leak and cardiac arrhythmias. Biochem Soc Trans 2007; 35: 952–6.
Huang F, Shan J, Reiken S, Wehrens XH, Marks AR . Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci USA 2006; 103: 3456–61.
Cheng YS, Dai DZ, Dai Y . Stress-induced cardiac insufficiency relating to abnormal leptin and FKBP12.6 is ameliorated by CPU0213, an endothelin receptor antagonist, which is not affected by the CYP3A suppressing effect of erythromycin. J Pharm Pharmacol 2009; 61: 569–76.
Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, et al. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res 2009; 81: 536–45.
Qi MY, Feng Y, Dai DZ, Li N, Cheng YS, Dai Y . CPU86017, a berberine derivative, attenuates cardiac failure through normalizing calcium leakage and downregulated phospholamban and exerting antioxidant activity. Acta Pharmacol Sin 2010; 31: 165–74.
Na T, Dai DZ, Tang XY, Dai Y . Upregulation of leptin pathway correlates with abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure and reversal by CPU86017. Naunyn Schmiedebergs Arch Pharmacol 2007; 375: 39–49.
Isaka M, Kudo A, Imamura M, Kawakami H, Yasuda K . Endothelin receptors, localized in sympathetic nerve terminals of the heart, modulate norepinephrine release and reperfusion arrhythmias. Basic Res Cardiol 2007; 102: 154–62.
Zheng JM, Zhu JM, Li LS, Liu ZH . Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br J Pharmacol 2008; 153: 1456–64.
Gao Q, Qin WS, Jia ZH, Zheng JM, Zeng CH, Li LS, et al. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med 2010; 76: 27–33.
Jia ZH, Liu ZH, Zheng JM, Zeng CH, Li LS . Combined therapy of rhein and benazepril on the treatment of diabetic nephropathy in db/db mice. Exp Clin Endocrinol Diabetes 2007; 115: 571–6.
Legendre F, Heuze A, Boukerrouche K, Leclercq S, Boumediene K, Galera P, et al. Rhein, the metabolite of diacerhein, reduces the proliferation of osteoarthritic chondrocytes and synoviocytes without inducing apoptosis. Scand J Rheumatol 2009; 38: 104–11.
Boileau C, Tat SK, Pelletier JP, Cheng S, Martel-Pelletier J . Diacerein inhibits the synthesis of resorptive enzymes and reduces osteoclastic differentiation/survival in osteoarthritic subchondral bone: a possible mechanism for a protective effect against subchondral bone remodelling. Arthritis Res Ther 2008; 10: R71.
Malaguti C, Vilella CA, Vieira KP, Souza GH, Hyslop S, Zollner Rde L . Diacerhein downregulate proinflammatory cytokines expression and decrease the autoimmune diabetes frequency in nonobese diabetic (NOD) mice. Int Immunopharmacol 2008; 8: 782–91.
Legendre F, Bogdanowicz P, Martin G, Domagala F, Leclercq S, Pujol JP, et al. Rhein, a diacerhein-derived metabolite, modulates the expression of matrix degrading enzymes and the cell proliferation of articular chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways. Clin Exp Rheumatol 2007; 25: 546–55.
Li N, Jia N, Dai DZ, Dai Y . Endothelin receptor antagonist CPU0213 and vitamin E reverse downregulation of FKBP12.6 and SERCA2a: a role of hyperphosphorylation of PKCepsilon. Eur J Pharmacol 2008; 591: 211–8.
Xia HJ, Dai DZ, Dai Y . Up-regulated inflammatory factors endothelin, NFkappaB, TNFalpha and iNOS involved in exaggerated cardiac arrhythmias in l-thyroxine-induced cardiomyopathy are suppressed by darusentan in rats. Life Sci 2006; 79: 1812–9.
Du RH, Yi HW, Dai DZ, Tang WH, Dai Y . Inflammatory factors that contribute to upregulation of ERG and cardiac arrhythmias are suppressed by CPU86017, a class III antiarrhythmic agent. J Pharm Pharmacol 2008; 60: 1089–95.
Altamirano E, Drut R . Arrhythmogenic cardiomyopathy in a patient with Noonan syndrome. Fetal Pediatr Pathol 2010; 29: 158–64.
Zumhagen S, Spieker T, Rolinck J, Baba HA, Breithardt G, Böcker W, et al. Absence of pathognomonic or inflammatory patterns in cardiac biopsies from patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2009; 2: 16–23.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No 81070145) and the National Key New Drug Innovation Program, Ministry of Science and Technology of China, No 2009ZX09308.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Gl., Dai, Dz., Xi, T. et al. Isoproterenol-induced FKBP12.6/12 downregulation is modulated by ETA and ETB receptors and reversed by argirhein, a derivative of rhein. Acta Pharmacol Sin 32, 223–229 (2011). https://doi.org/10.1038/aps.2010.177
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.177
Keywords
This article is cited by
-
Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats
Scientific Reports (2018)
-
Depressed calcium-handling proteins due to endoplasmic reticulum stress and apoptosis in the diabetic heart are attenuated by argirein
Naunyn-Schmiedeberg's Archives of Pharmacology (2013)
-
Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine
Journal of Biomedical Science (2012)
-
ER Stress, P66shc, and P-Akt/Akt Mediate Adjuvant-Induced Inflammation, Which Is Blunted by Argirein, a Supermolecule and Rhein in Rats
Inflammation (2012)