Abstract
Blood vessels have a fundamental role both in skeletal homeostasis and in bone repair. Angiogenesis is also important for a successful bone engineering. Therefore, scaffolds should be tested for their ability to favour endothelial cell adhesion, proliferation and functions. The type of endothelial cell to use for in vitro assays should be carefully considered, because the properties of these cells may depend on their source. Morphological and functional relationships between endothelial cells and osteoblasts are evaluated with co-cultures, but this model should still be standardized, particularly for distinguishing the two cell types. Platelet-rich plasma and recombinant growth factors may be useful for stimulating angiogenesis.
Similar content being viewed by others
Role of angiogenesis in bone engineering
In bone, the connection between cells and blood vessels is required to maintain skeletal integrity. In tissue engineering, a vessel network is an essential pre-requisite for scaffolds to survive and integrate with existing host tissue.
Activators and inhibitors of angiogenesis
Vascular development is a co-ordinated process through three major steps, regulating (1) sprouting of endothelial cells (ECs) from mature vessels, (2) assembly of vessels to vascular structures and (3) vessel maturation and subsequent induction of quiescence1. Each of these steps is regulated by molecules acting on specific vascular receptors. Sprouting is induced by vascular endothelial growth factor (VEGF)2, which is produced by monocytes and macrophages migrated to the site of the tissue lesion and stimulated by hypoxia. Vessel cells become sensitive to VEGF after the hypoxia-induced bond of angiopoietin-2 to the endothelial receptor tyrosine kinase Tie-2. VEGF binds to receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR) on EC membrane. Assembly of vessels to vascular structures is regulated by the ephrin ligands and ephrin receptor tyrosine kinases, which mediate cell-contact-dependent signalling3. Angiopoietins4 and Tie-1 and -2 receptors5 regulate blood vessel maturation too. Angiogenesis is also modulated by other growth factors (GFs), cytokines, enzymes and integrins, such as fibroblast growth factor 2 (FGF-2)6, hepatocyte growth factor/scatter factor7, platelet derived growth factor (PDGF)8, interluekin-8 (IL-8)9, IL-310, αvβ3-integrin11 and matrix metalloproteinases (MMPs)12, which degrade extracellular matrix (ECM) facilitating EC migration.
Angiogenesis and bone
In bone, microvessels are essential for bone formation, metabolism, healing and remodelling. Osteoprogenitors are always located near blood vessels. Sinusoids surrounded by reticular cells secrete high amounts of chemokine CXCL12 or stromal cell-derived factor-1 (SDF-1), which is required for the maintenance of human stem cells13. Both intramembraneous and endochondral bone ossification occur in close proximity to vascular ingrowth. In intramembranous ossification there is an invasion of capillaries that transport marrow stromal cells (MSCs), which differentiate into osteoblasts and in turn deposit bone matrix. In endochondral ossification the avascular cartilage template is replaced by highly vascularized bone tissue. The immature and proliferating chondrocytes secrete angiogenic inhibitors, while hypertrophic chondrocytes produce angiogenic stimulators, such as VEGF, fibroblast growth factor (FGF)-1 and FGF-2, thus providing a target for capillary invasion and angiogenesis. Hypertrophic chondrocytes and migrating cells from the newly formed bone marrow secrete metalloproteinases (MMPs), which in turn degrade extracellular matrix (ECM), thus permitting vessel invasion. MMP-9 regulates also the release of VEGF-A bound to the hypertrophic cartilage matrix. Once released, VEGF-A binds to its receptors on endothelial cells, osteoclasts and osteoblasts14. The new vasculature supplies a conduit for the recruitment of cells involved in cartilage resorption and bone deposition15. ECs produce GFs which contribute to recruit stem cells and to address them towards osteoblast differentiation.
Blood vessels play a crucial role in both phases of bone remodelling. In bone resorption, vessels transport osteoclast precursors to the sites of remodelling16. In bone deposition, vessels transport osteoprogenitor cells17. According to their phenotype, ECs produce molecules modulating bone remodelling, such as RANKL, osteoprotegerin (OPG), IL-6, PDGF, transforming growth factor-β (TGF-β), and others.
Angiogenesis is fundamental for fracture repair. One of the earliest events during bone healing is the reconstruction of intraosseous circulation18. Following trauma, disruption of vessels leads to acute hypoxia of the surrounding tissue, as well as to clotting activation. The inflammatory response activates cytokines and GFs that recruit MSCs and ECs to the fracture site. The latter produce PDGF-BB, which contributes to MSC recruitment19. Lack of angiogenesis is considered as a pathogenetic cause of non-unions20.
Angiogenic GFs also play roles in bone formation. VEGF seems to play a key role in endochondral ossification21, where its functions are mediated by cbfa-1/runx-222. VEGF production is increased by BMP-2, -4, and -623, and by TGF-β124. VEGF-A binds to VEGFR-1 on osteoclasts and induce osteoclast recruitment and bone-resorption25. FGF-2 stimulates the proliferation and differentiation of osteoblasts26 and accelerates fracture repair when added to the early healing stage27.
Relationships between endothelial cells and osteoblasts
ECs and osteoblasts (OBs) communicate through three mechanisms28:
1. Direct interaction between membrane molecules of the two adjacent cells (tight junctions);
2. Gap junction communications that form direct cytoplasmic connections between adjacent cells;
3. Secretion of diffusible factors that diffuse freely in the extracellular environment and interact with the target cells through specific receptors.
Gap junction communications are mediated by Cx43 on OBs and ECs, and by Cx40 on ECs29, 30.
Some diffusible factors secreted by ECs favour bone deposition, such as PDGF-AB, TGF-β1, TGF–β2, FGF-2, epidermal growth factor (EGF), OPG, and bone morphogenetic protein 2 (BMP-2)31. When ECs are incubated with a proinflammatory stimulus, such as TNF, IL-1β, or endotoxin, they synthesize both substances inducing bone healing, such as endothelin-132, and molecules favouring bone resorption, such as IL-6 and RANK-L33. Moreover, ECs may stimulate osteoblasts to express ALP34.
In turn, MSCs produce angiogenic GFs, such as VEGF, particularly under hypoxic conditions, FGF-2, insulin-like growth factors (IGF), PDGF, and TGF-β. VEGF-stimulated ECs release prostaglandins that strongly promote VEGF release by osteoblasts35. ECs co-cultured with OBs show an increased production of collagen type I36.
The interaction between ECs and OBs is variable with time, as it was shown in co-cultures onto a scaffold made of starch and polycaprolactone. At early time points ECs formed monolayer patches above OBs. At 21 days, ECs had organized into microcapillary-like structures, which were established among OBs. The concentration profile of VEGF during 35 days in vitro was characterized by 3 distinct phases: (1) from day 7 to 14 a steep increase in VEGF concentration; (2) between day 14 and day 28 a plateau phase and (3) from day 28 until day 35 a pronounced decrease of VEGF concentration. In hOB monoculture, the VEGF concentration curve exhibited a steady increase at lower magnitude as compared to co-culture until day 28 followed by a decrease37.
The sonic hedgehog (Shh) pathway is involved both in bone repair and in neoangiogenesis. Hedgehog morphogens play a pivotal role in embryonic development38. There is increasing evidence that the Shh pathway plays a significant role in adults both in angiogenesis39, 40 and in endochondral bone formation41.
Endothelial cells
ECs are classified into macrovascular and microvascular, according to the vessel type.
Human umbilical vein endothelial cells and other macrovascular endothelial cells
HUVEC are the most known among macrovascular ECs. Other macrovascular ECs were isolated from human saphena or from human, bovine or swine aorta or pulmonary artery. However, the latter EC types have been scarcely used for the evaluation of scaffolds for bone engineering. Moreover, non-human ECs show a different behaviour than human cells42.
Microvascular endothelial cells
There is evidence that ECs from different organs exhibit different responses to stimulants - particularly, macrovascular ECs have different properties from microvascular ECs.
Microvascular endothelial cells (HMVEC) were isolated from adipose tissue (ADEC)43, derma (HDMEC)44 or lung microvessels (HPMEC)45. The advantages of ADEC or HDMEC consist in more similar properties to bone microvessels than HUVEC. Moreover, in light of a possibile clinical application, they may be easily isolated from the same patient who will receive the scaffold.
Endothelial progenitor cells
EPCs are adult progenitor cells that can differentiate into mature ECs46 and therefore play a physiological role in vessel homeostasis47. EPCs may be identified through the expression of three cell markers (CD133, CD34, and VEGFR-2)48. EPCs are mainly located in bone marrow and can be mobilized into peripheral blood49, where they are present from 0.01% to 0.0001% of mononuclear cells (MNCs) in healthy subjects50. In culture, two distinct types of EPCs develop. The first type, named early EPCs51, appears after 3–5 days, is formed by spindle-shaped cells and dies after 4 weeks. The second type, named late EPCs52 or outgrowth endothelial cells (OECs)51, appears after 2–3 weeks, forms a cobblestone monolayer and lives for about 12 weeks. Early EPCs, which derive from CD14+ MNCs, are myeloid cells with some endothelial properties, which stimulate neovascularization by paracrine factors but are not incorporated in the endothelial lining. OECs derive from CD14- MNCs, have similar properties to mature ECs but a higher proliferative ability53, and are incorporated into the endothelial lining of new blood vessels54.
One of the most therapeutically interesting features of EPCs is their apparently enhanced ability to be incorporated into newly forming microvasculature. Although their concentration in blood is low, they have been detected in newly formed vasculature, contributing about 5%–35% of the endothelial cells in new capillaries55. In fact, EPCs are mobilized by tissue ischemia and cytokines from the bone marrow into peripheral blood, migrate to regions of neovascularization, differentiate into mature endothelial cells and promote vasculogenesis56. The most known application of EPCs is the promotion of the therapeutic neovascularization in myocardial infarction57 and liver disorders58. Moreover, it was shown that EPCs develop a favorable environment for fracture healing via angiogenesis and osteogenesis, through two mechanisms. One is the osteogenic and endothelial differentiation potential of CD34+ cells, and the other one is the paracrine effect of CD34+ cells, which secrete VEGF59. For this reason, EPCs were investigated to specifically address the problem of delayed and atrophic non-unions60.
Endothelial cell continuous cell lines
Typically, EC cultures are primary cultures and the proliferative potiential gradually decreases during passages. Therefore continuous cell lines were generated from angiosarcomas, or through cell immortalization with viral transfection or with fusion with neoplastic lines. Examples of tumour-derived endothelial cell lines are ISO-HAS from human haemangiosarcoma61 and HAEND derived from hepatic angiosarcoma62. Transfection may be obtained with SV40 virus or with the introduction of human telomerase reverse transcriptase (hTERT)63. Examples of lines obtained with transfection are EVLC2, derived from HUVEC64, HMEC-1, developed by transfection of HDMEC with SV-40 large T-antigen65, and HPMEC-ST1.6R, developed by transfection of HPMEC with plasmids encoding the SV-40 large T-antigen and human telomerase66.
A line obtained from the fusion of ECs with neoplastic cells is EA.hy926, which was developed from the fusion of HUVEC with human pulmonary adenocarcinoma A54967.
The changes resulting in the capacity of cells to replicate indefinitely may be accompanied by changes in the expression of specific endothelial properties, such as the induction of inter-cellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) or E-selectin with an inflammatory stimulus68. Among EA.hy926, EVLC2, HAEND, HMEC-1, ISOHAS-1, only HPMEC-ST1.6R exhibited the major constitutive and inducible endothelial cell characteristics and showed an angiogenic response on Matrigel69.
A continuous EC line was isolated from the microvessels of bovine foetal sternus70. The endothelial phenotype was shown by the presence of von Willebrand factor and by the ability to form tubular-like structures on Matrigel. These cells display own distinctive characteristics, particularly they possess the receptor for estrogens and are able to respond to estrogens and parathyroid hormone (PTH)71, 72, 73.
Evaluation of the angiogenic potential of the scaffold
Upon graft implantation, inflammation, which represents the first phase of tissue repair, favours a vascular response, but angiogenesis is generally limited to less than 1 mm from the interface implant-host tissue74. Moreover, the capillary network induced by the inflammatory process is transient and regress within a few weeks75. Neoformed vessels of the implant must anastomose to the systemic circulation76. In the absence of a vascular supply, the transport of nutrients occurs mainly by diffusion, which is only efficient for distances from 100 to 200 micron or for tissues with a low metabolic activity, such as cartilage77. The insufficient vascularization compromises the supply of oxygen and nutrients to the new-formed tissue and does not remove the waste products of cells78. The local accumulation of toxic substances may trigger an inflammatory reaction79.
Therefore the scaffold must not only support the growth of the cells that will replace the specific tissue in vivo, but it must also support EC adhesion and proliferation, and develop an effectively functioning vasculature to supply the cells with oxygen and nutrients. The success of this strategy requires a series of consecutive events: a) EC migration to the outer surface of the scaffold, b) EC migration from the outer surface to the inner pores of the scaffold, c) EC adhesion to the foreign surface and proliferation, d) ECM synthesis, e) tubular structures formation, f) recruitment of further cell types (smooth muscle cells, pericytes, fibroblasts) forming the vessel wall and g) anastomosis with the vessels of the surrounding tissue (Figure 1). Moreover, endothelial cells growing on the scaffold should maintain normal functions and should not exhibit a pro-inflammatory phenotype. In order to obtain stable and durable vascular networks, ECs require the cooperation with perivascular cells. It was shown that a network of stable and functional blood vessels was formed in mice by co-implantation of vascular ECs and mesenchymal precursor cells80.
In the light of the critical role of angiogenesis, a preliminary step in the evaluation of the scaffold properties should predict its vascularization potential through the assessment of its interaction with ECs.
Endothelial cell seeding
ECs are seeded on the scaffold, which may be pre-conditionated by immersion in culture medium for a few hours. This treatment leads to protein adsorption on the artificial surface, and purposes to reproduce the ECM layer to which the cells usually adhere to live on. Cell seeding is favoured by the following technique: to apply a drop of cell suspension on the scaffold, to incubate at 37 °C for 15–30 min in a wet chamber to allow initial cell attachment, then to add culture medium. The scaffold may be coated with gelatin or fibronectin or collagen or pooled serum to favour cell adhesion.
Endothelial cell morphology
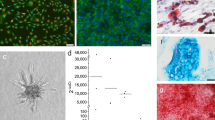
Cell morphology can be evaluated before or after staining. Immunofluorescence for specific antigens, such as von Willebrand factor or CD31, or stain with fluorescent molecules, such as calcein-acetoxymethylester81 or acridine orange, may be used. Cytoskeleton is shown by F-actin stain (Figure 2A) or by immunofluorescence for vimentin82. VE-cadherin is an adhesion molecule that mediates cell-to-cell contact between endothelial cells and plays a relevant role in the maintenance of vascular integrity83 (Figure 2B). The maintenance of VE-cadherin expression on a scaffold is a good indicator of the proper interaction between endothelial cells and the material. Fluorescent staining of intracellular organelles may be evaluated by confocal microscopy. With fluorescence microscopy and scanning electron microscopy the relationships between cells and scaffold may be investigated, but no information is obtained on the biomaterial effects on the synthesis of specific molecules.
Immunofluorescent staining of endothelial cells for proteins specific for cytoskeleton or for adhesion molecules. (A) F-actin stain of HUVEC. Actin was stained using rhodamine-phalloidin fluorescent dye. Nuclei were stained with Hoechst 33258. Magnification (×20). (B) Immunofluorescent staining for VE-cadherin. Nuclei were stained with Hoechst 33258. Magnification (×20).
Adhesion and spreading
The scaffold could affect the synthesis of molecules for homotypic adhesion (CD31, VE-cadherin) or of integrin for the adhesion to substratum84. To favour EC and osteoblast adhesion, RGD-peptides were bound to the scaffold. These peptides are formed by the arginine, glutammic acid and aspartic acid sequence, which is common to the integrin αvβ3 ligands85. Moreover, the scaffold could decrease the expression of adhesion molecules for leukocytes (E-selectin, ICAM and VCAM) in response to inflammatory stimula or, vice versa, could induce their expression also on basal conditions86. Cell adhesion to the substratum and adhesion molecules are evaluated at fluorescence and confocal microscopy with immunofluorescence staining87.
A quantitative method to evaluate the expression of adhesion molecules is an enzyme immunoassay performed directly on the cells adherent to the substratum (CAM-EIA)88. Flow cytometry is another quantitative assay for adhesion molecules and integrins, but it requires cell detachment, which could affect their expression89. Moreover, adhesion molecules and integrins may be indirectly evaluated with RT-PCR for their specific mRNA90.
Cell proliferation
The proliferation of endothelial cells on artificial scaffolds may be directly determined with vital stains, such as blue alamar91 or calcein-acetoxymethylester92, without detaching cells. Alamar changes from blue to pink in proportion to the amount of reactions of oxide-reduction of the cells and therefore in proportion to the cell number. Through a standard curve with known cell numbers, the cell number of the unknown sample grown on the scaffold can be obtained with a good approximation93. Calcein-acetoxymethylester becomes fluorescent when taken up by viable cells and the fluorescence is spread throughout the cell. Alternatively the cell number can be evaluated with tritiated thymidine94, MTT95, or crystal violet96 assays.
Tubulogenesis
EC grown on the scaffold can be evaluated for their ability to form tubular-like structures. ECs are seeded on an inducing matrix, with or without GFs. This matrix can be used also to coat the scaffold. After a few hours, tubular-like structure formation is observed, eventually after vital staining with calcein-acetoxymethylester and nuclear staining with Hoechst 3334297. The tube number, length and bifurcations may be quantificated with image analysis, in order to appreciate differences among scaffolds. Matrigel (Becton Dickinson), formed by EMC proteins, is a well-known matrix. Also type I collagen was used, which determined after 20 h the formation of tubular-like structures similar to capillaries97.
Expression of proteins acting on bone remodelling
For the evaluation of scaffolds for bone engineering, the assay of GFs, cytokines and other proteins produced by endothelium and acting on bone remodelling could be useful. Both specific mRNA expression98 and the concentration of these substances in the conditioned medium are determined99. Proinflammatory markers should be assayed both on basal conditions and after incubation with LPS, because the biomaterial could affect the cell response to the proinflammatory stimulus100.
Co-cultures
The different co-culture systems take the different interactions among ECs and OBs into account (Figure 3):
Cell co-culture systems. In the direct contact model, cells are seeded together in 2D supports or 3D scaffolds or as 3-D multicellular spheroids. In the indirect contact model, a porous membrane can be used, with appropriate pore size which let the conditioned medium pass but not cells. Alternatively, the culture of one type of cells is supplemented with the conditioned medium of the other type. In the third method, one cell type is seeded on the ECM of the other type, which has been discharged after grown.
- Co-cultures with direct contact can be initiated on a 2-D surface or on a 3D scaffold or with spheroid systems. With direct contact all the mechanisms of cell-to-cell interaction are evaluated (direct interaction through tight junctions, gap junction communications and secretion of paracrine factors)28;
- Co-cultures with indirect contact evaluate only communications through paracrine soluble factors. The two cell types may be put in indirect contact through a porous membrane with a such pore size which lets the conditioned medium pass but not cells101. Other methods consist in culturing one cell type in the conditioned medium of the other type or in seeding a cell type over the ECM that has been produced by the other cell type.
The indirect contact methods allow to easily quantificate the reciprocal metabolic influences, but do not give any information on the reciprocal spatial relationships.
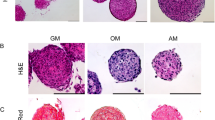
With the direct contact method the relationships between cells into the living tissue are simulated. 2D studies provide detailed information of the molecular basis of cell-to-cell contacts, and knowledge of cellular events governing the differentiation of OBs that are in contact with ECs. Conversely, 3D co-cultures offer a physiologically optimized environment for cell survival which favors the formation of functional blood vessels. Spheroids formed when a cellular suspension in medium containing 20% methyl cellulose (Methocel, Dow Chemical Co, USA) was seeded in nonadhesive wells with U shape102. HUVECs were grown as 3-D multicellular spheroids in a collagen matrix. Direct cell contact between hOBs and HUVECs was established by incorporating hOBs into the EC spheroids, thus forming heterogeneous cospheroids. Co-culture spheroids differentiated spontaneously to organize into a core of OBs and a surface layer of ECs103.
Direct contact method requires the standardization of a) the choice of culture medium, b) the choice to seed the cell types at the same time or successively, and which type should be seeded as the first; c) the ratio between cell types, d) the separation of the cell types during and at the end of the co-cultures. As culture medium, the medium of ECs, which have higher nutrition requirements, is usually chosen. Both cell types may be seeded at the same time or ECs are seeded before OBs. The optimal ratio between HDMVEC and OBs was shown to be 5:1 or 10:192. The evaluation of the relationships between the cell types is based on differential staining with quantum dots incorporated by cells before seeding and the evaluation at fluorescence or confocal microscopy. Also staining for von Willebrand factor or CD31, specific for ECs, and ALP, specific for OBs, were used. Time-lapse microscopy showed the formation of a tubular-like network through the movement of HUVEC along hOB and their philopodia104.
With morphological methods the relationships between cells are investigated, but no information on the reciprocal metabolic influences is obtained. The latter is examined with the assay of specific genes and protein of each cell type. ECs produce VEGFR-1, VEGFR-2, CD31, Tie-1, Tie-2, but do not synthesize collagen I, ALP and osteocalcin. ECs co-cultured with hOB stimulated ALP activity and mineralization105, but down-regulated runx2, osteocalcin and Cx43106. The most serious problem is the evaluation of the relative synthesis of proteins because the total protein content of each cell type cannot be distinguished. Also gene expression cannot be normalized because housekeeping genes are common to hECs and hOB, unless human and animal cells are co-cultured. Therefore it is fundamental to separate the two cell types at the end of co-culture. Magnetic immunoseparation with anti-CD31 antibodies showed upregulation of 79 genes and downregulation of 62 genes in OBs and, particularly, the downregulation of the gene of PDGF receptor α after co-culture with ECs101.
Direct co-culture of ECs and OBs prevented the precoating of biomaterials with gelatin or fibronectin. Moreover, ECs formed an extensive network of capillary-like structures with lumina only when they were co-cultured with OBs, but not when they were cultured on biomaterials alone even in the presence of an exogenously supplied angiogenic stimulus. Thus, a prevascularization can take place in vitro only in the presence of OBs92.
Local delivery of angiogenic growth factors
Biomaterial vascularization can be promoted or by seeding mature ECs or their progenitors directly on scaffolds107, or by locally delivering angiogenic GFs108, which may induce ECs to migrate, proliferate, and produce molecules acting on bone remodelling. Particularly, it was shown that VEGF-A increased mRNA specific for FGF-2 and decreased mRNA specific for IL-619, and increased both mRNA expression and surface protein expression of RANK109.
Delivery of recombinant growth factors
The ability of biodegradable scaffolds to locally deliver GFs mimicks the conditions of tissue repair in vivo. A combination of PDGF-BB and VEGF-165 initiated formation and maturation of a significant number of blood vessels110. When calvarial defects of rats were treated with scaffolds in poly(D,L-lactide-co-glycolide) (PLGA) bound to rhVEGF-A, a significant increase of blood vessel formation, bone coverage, and bone mineral density was observed in comparison with defects treated with simple PLGA111. The use of rhVEGF-A in bone defect models showed that new blood vessel formation preceded the osteogenic front and that an increased angiogenesis corresponded to an increased bone formation112. However, the local application of VEGF-A to rabbit tibia during distraction osteogenesis increased the blood flow in the distracted limb, but failed to influence bone mineral content and histomorphometric indices of bone regeneration113. A possible explanation was that a high level of endogenous VEGF-A had already been secreted during osteogenesis, reaching an optimal local concentration, and therefore the additional delivery of VEGF-A had little or no effect114.
Platelet-rich plasma (PRP)
A more feasible way to administer angiogenic GFs consists in the application of PRP. In fact, activated platelets release osteogenic and angiogenic GFs from α-granules, such as PDGF, TGF-β, IGF, EGF, and VEGF. Therefore autologous platelets activated with thrombin were used as a source of GFs to stimulate tissue repair. PRP could also favour proliferation and differentiation of the cells seeded on scaffolds, ECs included115, also when they were co-cultured with hOB116.
Gene therapy
Ex vivo gene therapy, which consists in the transplantation of genetically modified MSCs secreting angiogenic GFs117, could overcome the limits of conventional GF delivery. When adipose-derived stem cells (ADSCs) were transfected with adenovirus encoding the cDNA of VEGF, the combination of VEGF releasing cells and ECs resulted in a higher vascular growth within PLGA scaffolds118.
Discussion
In the last forty years, the new knowledge in cellular and molecular biology and the possibility of the synthesis of innovative materials have determined a shift from the concept of an “inert” material, ie non-toxic for cells and tissues of the body, to the concept of a “bioactive” material, which favours cellular adhesion, proliferation and functions. At the same time, it has been understood that angiogenesis was necessary not only for the treatment of obstructive vasculopathies, but also for the repair of most tissues and organs. Consequently, it has been understood that the successful clinical outcome of an implanted cell-construct is dependent on the establishment of a functional vascular network. Therefore, scaffolds should be tested for their angiogenic potential before implantation. Particularly, the ability to favour EC adhesion, proliferation and functions should be assayed with in vitro and in vivo tests.
The choice of the EC type is crucial, because they may have different properties according to their source. For the evaluation of scaffolds intended for bone engineering, cells with similar characteristics to ECs of bone vasculature should be chosen. Olfactory ensheathing cells (OECs), which can be isolated from the patients without invasive methods, seem to be the most suitable in the formation of functional vessels anastomosed to the host's vascular system119.
Among the different models of in vitro evaluation of the angiogenic potential, the co-cultures between ECs and OBs are the closest to the in vivo situation. However, they allow to appreciate the reciprocal relationships between these cell types, but still need to be standardized for the quantitative evaluation of specific gene expression and protein synthesis.
At present, in vitro models alone cannot predict if the capillary-like structures pre-formed in the scaffold will establish connections in vivo with the host microvascular system. A co-culture of EPCs and MSCs on a scaffold followed by implantation in animal demonstrated improved osteogenesis and angiogenesis when the scaffold had been seeded with the two cell types, without ischemic necrosis at the center of the graft, while impaired osteogenesis and progressive necrosis were observed when the scaffold had been seeded with only OBs120.
At present, efforts are mainly focused on stabilizing neovasculature and thus promoting the formation of lasting blood vessels. Perivascular cells such as pericytes and smooth muscle cells contribute to the remodelling and maturation of the primitive vascular network and therefore are fundamental agents in the construction of a durable engineered vasculature. In this line of thought, the actual co-culture systems will be upgraded with tri-cultures among OBs, ECs and perivascular cells.
In conclusion, blood vessels, which are necessary to skeletal homeostasis and bone repair, have a fundamental role to assure the incorporation of a cells-scaffold construct into the body. Therefore, the ability of the scaffolds to favour EC adhesion and proliferation, without affecting their functions, should be assayed. In in vitro tests, the source of ECs should be carefully considered, because it may affect their properties. Morphological and functional relationships between ECs and OBs should be evaluated with appropriate models, such as co-cultures. PRP and recombinant GFs may be useful for stimulating neoangiogenesis.
Author contribution
Elisabetta CENNI wrote the manuscript; Francesca PERUT contributed to the sections Endothelial progenitor cells, Evaluation of the angiogenic potential of the scaffold, and Local delivery of angiogenic growth factors, and to the photographs at fluorescence microscopy; Nicola BALDINI supervised the paper.
References
Polykandriotis, Arkudas A, Horch RE, Stürzl M, Kneser U . Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med 2007; 11: 6–20.
Ferrara N, Gerber HP, LeCouter J . The biology of VEGF and its receptors. Nat Med 2003; 9: 669–76.
Heroult M, Schaffner F, Augustin HG . Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res 2006; 312: 642–50.
Thurston G . Role of angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res 2003; 314: 61–8.
Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, et al. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res 2004; 59: 51–71.
Przybylski M . A review of the current research on the role of bFGF and VEGF in angiogenesis. J Wound Care 2009; 18: 516–9.
You WK, McDonald DM . The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep 2008; 41: 833–9.
Andrae J, Gallini R, Betsholtz C . Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008; 22: 1276–312.
Waugh DJ, Wilson C . The interleukin-8 pathway in cancer. Clin Cancer Res 2008; 14: 6735–41.
Zeoli A, Dentelli P, Rosso A, Togliatto G, Trombetta A, Damiano L, et al. Interleukin-3 promotes expansion of hemopoietic-derived CD45+ angiogenic cells and their arterial commitment via STAT5 activation. Blood 2008; 112: 350–61.
Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, et al. Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis. J Biol Chem 2009; 284: 33966–81.
Davis GE, Senger DR . Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 2005; 97: 1093–107.
Sugiyama T, Kohara H, Noda M, Nagasawa T . Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–88.
Nakagawa M, Kaneda T, Arakawa T, Morita S, Sato T, Yomada T, et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett 2000; 473: 161–4.
Harper J, Klagsbrun M . Cartilage to bone-angiogenesis leads the way. Nat Med 1999; 5: 617–8.
Kanczler JM, Oreffo RO . Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 2008; 15: 100–14.
Barou O, Mekraldi S, Vico L, Boivin G, Alexandre C, Lafage-Proust MH . Relationships between trabecular bone remodeling and bone vascularization: a quantitative study. Bone 2002; 30: 604–12.
Schindeler A, McDonald MM, Bokko P, Little DG . Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol 2008; 19: 459–66.
Cenni E, Ciapetti G, Granchi D, Fotia C, Perut F, Giunti A, et al. Endothelial cells incubated with platelet-rich plasma express PDGF-B and ICAM-1 and induce bone marrow stromal cell migration. J Orthop Res 2009; 27: 1493–8.
Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Mineral Res 2005; 20: 1114–24.
Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N . VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 1999; 5: 623–8.
Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev 2001; 106: 97–106.
Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW . Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000; 141: 1667–74.
Chang SC, Chuang HL, Chen YR, Chen JK, Chung HY, Lu YL, et al. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther 2003; 10: 2013–9.
Niida S, Kondo T, Hiratsuka S, Hayashi S, Amizuka N, Noda T, et al. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci USA 2005; 102: 14016–21.
Pun S, Dearden RL, Ratkus AM, Liang H, Wronski TJ . Decreased bone anabolic effect of basic fibroblast growth factor at fatty marrow sites in ovariectomized rats. Bone 2001; 28: 220–6.
Chen WJ, Jingushi S, Aoyama I, Anzai J, Hirata G, Tamura M, et al. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J Bone Miner Metab 2004; 22: 303–9.
Grellier M, Bordenave L, Amédée J . Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol 2009; 27: 562–71.
Yeh HI, Lee PY, Su CH, Tian TY, Ko YS, Tsai CH . Reduced expression of endothelial connexins 43 and 37 in hypertensive rats is rectified after 7-day carvedilol treatment. Am J Hypertens 2006; 19: 129–35.
Villars F, Guillotin B, Amedee T, Dutoya S, Bordenave L, Bareille R, et al. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol 2002; 282: C775–C785.
Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg 2002; 109: 2384–97.
von Schroeder HP, Veillette CJ, Payandeh J, Qureshi A, Heersche JN . Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone 2003; 33: 673–84.
Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P . Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem 2001; 276: 20659–72.
Rouwkema J, De Boer J, van Blitterswijk CA . Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 2006; 12: 2685–93.
Clarkin CE, Emery RJ, Pitsillides AA, Wheeler-Jones CP . Evaluation of VEGF mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J Cell Physiol 2008; 214: 537–44.
Fuchs S, Jiang X, Schmidt H, Dohle E, Ghanaati S, Orth C, et al. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials 2009; 30: 1329–38.
Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ . Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 2009; 30: 4407–15.
Nagase T, Nagase M, Machida M, Yamagishi M . Hedgehog signaling: a biophysical or biomechanical modulator in embryonic development? Ann N Y Acad Sci 2007; 1101: 412–38.
Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001; 7: 706–11.
Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ . Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A 2010; 16: 1235–7.
van der Horst G, Farih-Sips H, Lowik CW, Karperien M . Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone 2003; 33: 899–910.
Pasquinelli G, Freyrie A, Preda P, Curti T, D'Addato M, Laschi R . Healing of prosthetic arterial grafts. Scanning Microsc 1990; 4: 351–62.
Perego G, Preda P, Pasquinelli G, Curti T, Freyrie A, Cenni E . Functionalization of poly-(L-lactic-co-epsilon-caprolactone): effects of surface modification on endothelial cell proliferation and hemocompatibility. J Biomater Sci Polym Ed 2003; 14: 1057–75.
Rouwkema J, Westerweel PE, de Boer J, Verhaar MC, van Blitterswijk CA . The use of endothelial progenitor cells for prevascularized bone tissue engineering. Tissue Eng Part A 2009; 15: 2015–27.
Krump-Konvalinkova V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ . Generation of human pulmonary microvascular endothelial cell lines. Lab Invest 2001; 81: 1717–27.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7.
Sirker AA, Astroulakis ZMJ, Hill MJ . Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci 2009; 116: 283–99.
Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000; 95: 952–8.
Korbling M, Reuben JM, Gao H, Lee BN, Harris DM, Cogdell D, et al. Recombinant human granulocyte-colony-stimulating factor-mobilized and apheresis-collected endothelial progenitor cells: a novel blood cell component for therapeutic vasculogenesis. Transfusion 2006; 46: 1795–802.
Khan SS, Solomon MA, McCoy JP Jr . Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom 2005; 64: 1–8.
Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 2003; 93: 1023–5.
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004; 24: 288–93.
Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005; 112: 1618–27.
Verloop RE, Koolwijk P, Zonneveld AJ, Hinsbergh VW . Proteases and receptors in the recruitment of endothelial progenitor cells in neovascularization. Eur Cytokine Netw 2009; 20: 207–19.
Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, et al. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 2002; 30: 967–72.
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5: 434–8.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–6.
Lemoli RM, Catani L, Talarico S, Loggi E, Gramenzi A, Baccarani U, et al. Mobilization of bone marrow-derived hematopoietic and endothelial stem cells after orthotopic liver transplantation and liver resection. Stem Cells 2006; 24: 2817–25.
Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol 2006; 169: 1440–57.
Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 2008; 43: 434–9.
Masuzawa M, Fujimura T, Hamada Y, Fujita Y, Hara H, Nishiyama S, et al. Establishment of a human hemangiosarcoma cell line (ISO-HAS). Int J Cancer 1999; 81: 305–8.
Hoover ML, Vetvicka V, Hoffpauir JM, Tamburro CH . Human endothelial cell line from an angiosarcoma. In Vitro Cell Dev Biol 1993; 29A: 199–202.
Shao R, Guo X . Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun 2004; 321: 788–94.
van Leeuwen EB, Veenstra R, van Wijk R, Molema G, Hoekstra A, Ruiters MH, et al. Characterization of immortalized human umbilical and iliac vein endothelial cell lines after transfection with SV40 large T-antigen. Blood Coagul Fibrinolysis 2000; 11: 15–25.
Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, et al. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992; 99: 683–90.
Krump V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ . Generation of human pulmonary microvascular endothelial cell lines. Lab Invest 2001; 81: 1717–27.
Edgell CJ, Haizlip JE, Bagnell CR, Packenham JP, Harrison P . Wilbourn B, et al. Endothelium specific Weibel–Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell Dev Biol 1990; 26: 1167–72.
Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, Martin A, et al. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: A comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest 2000; 80: 37–45.
Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ . In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res 2002; 64: 384–97.
Streeten EA, Ornberg R, Curcio F, Sakaguchi K, Marx S, Aurbach GD et al. Cloned endothelial cells from fetal bovine bone. Proc Natl Acad Sci USA 1989; 86: 916–20.
Streeten EA, Brandi ML . Biology of bone endothelial cells. Bone Miner 1990; 10: 85–94.
Brandi ML, Crescioli C, Tanini A, Frediani U, Agnusdei D, Gennari C . Bone endothelial cells as estrogen targets. Calcif Tissue Int 1993; 53: 312–7.
Fiorelli G, Orlando C, Benvenuti S, Franceschelli F, Bianchi S, Pioli P, et al. Characterization, regulation, and function of specific cell membrane receptors for insulin-like growth factor I on bone endothelial cells. J Bone Miner Res 1994; 9: 329–37.
Colton CK . Implantable biohybrid artificial organs. Cell Transplant 1995; 4: 415–36.
Sieminski AL, Gooch KJ . Biomaterial-microvasculature interactions. Biomaterials 2000; 21: 2232–41.
Scheufler O, Schaefer DJ, Jaquiery C, Braccini A, Wendt DJ, Gasser JA, et al. Spatial and temporal patterns of bone formation in ectopically pre-fabricated, autologous cell-based engineered bone flaps in rabbits. J Cell Mol Med 2008; 12: 1238–49.
Rouwkema J, Rivron NC, van Blitterswijk CA . Vascularization in tissue engineering. Trends Biotechnol 2008; 26: 434–41.
Hutmacher DW . Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000; 21: 2529–43.
Taylor MS, Daniels AU, Andriano KP, Heller J . Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater 1994; 5: 151–7.
Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK . Tissue engineering: creation of long-lasting blood vessels. Nature 2004; 428: 138–9.
Thimm BW, Unger RE, Neumann HG, Kirkpatrick CJ . Biocompatibility studies of endothelial cells on a novel calcium phosphate/SiO2-xerogel composite for bone tissue engineering. Biomed Mater 2008; 3: 015007.
Silva Marques JM, Gomes PS, Silva MA, Silvério Cabrita AM, Santos JD, Fernandes MH . Growth and phenotypic expression of human endothelial cells cultured on a glass-reinforced hydroxyapatite. J Mater Sci Mater Med 2009; 20: 725–31.
Mochizuki N . Vascular integrity mediated by vascular endothelial cadherin and regulated by sphingosine 1-phosphate and angiopoietin-1. Circ J 2009; 73: 2183–91
Amato I, Ciapetti G, Pagani S, Marletta G, Satriano C, Baldini N, et al. Expression of cell adhesion receptors in human osteoblasts cultured on biofunctionalized poly-(epsilon-caprolactone) surfaces. Biomaterials 2007; 28: 3668–78.
Marletta G, Ciapetti G, Satriano C, Pagani S, Baldini N . The effect of irradiation modification and RGD sequence adsorption on the response of human osteoblasts to polycaprolactone. Biomaterials 2005; 26: 4793–804.
Campillo-Fernández AJ, Unger RE, Peters K, Halstenberg S, Santos M, Salmerón Sánchez M, et al. Analysis of the biological response of endothelial and fibroblast cells cultured on synthetic scaffolds with various hydrophilic/hydrophobic ratios: influence of fibronectin adsorption and conformation. Tissue Eng Part A 2009; 15: 1331–41.
Jabbarzadeh E, Jiang T, Deng M, Nair LS, Khan YM, Laurencin CT . Human endothelial cell growth and phenotypic expression on three dimensional poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Biotechnol Bioeng 2007; 98: 1094–102.
Perut F, Cenni E, Unger RE, Kirkpatrick CJ, Giunti A, Baldini N . Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int J Oncol 2009; 34: 1387–93.
Cenni E, Granchi D, Verri E, Remiddi G, Cavedagna D, Di Leo A . Evaluation of endothelial cell integrins after in vitro contact with polyethylene terephthalate. J Mater Sci Mater Med 2001; 12: 345–9.
Williamson MR, Woollard KJ, Griffiths HR, Coombes AG . Gravity spun polycaprolactone fibers for applications in vascular tissue engineering: proliferation and function of human vascular endothelial cells. Tissue Eng 2006; 12: 45–51.
Deb S, Mandegaran R, Di Silvio L . A porous scaffold for bone tissue engineering/45S5 Bioglass derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci Mater Med 2010; 21: 893–905.
Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M, et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials 2007; 28: 3965–76.
Larson EM, Doughman DJ, Gregerson DS, Obritsch WF . A new, simple, nonradioactive, nontoxic in vitro assay to monitor corneal endothelial cell viability. Invest Ophthalmol Vis Sci 1997; 38: 1929–33.
Hopper RA, VerHalen JP, Tepper O, Mehrara BJ, Detch R, Chang EI, et al. Osteoblasts stimulated with pulsed electromagnetic fields increase HUVEC proliferation via a VEGF-A independent mechanism. Bioelectromagnetics 2009; 30: 189–97.
Qian YF, Zhang KH, Chen F, Ke QF, Mo XM . Cross-linking of gelatin and chitosan complex nanofibers for tissue-engineering scaffolds. J Biomater Sci Polym Ed 2010 doi: 10.1163/092050610x499447.
Lippert C, Seeger H, Wallwiener D, Mueck AO . Comparison of the effects of 17alpha-ethinylestradiol and 17beta-estradiol on the proliferation of human breast cancer cells and human umbilical vein endothelial cells. Clin Exp Obstet Gynecol 2002; 29: 87–90.
Peters K, Schmidt H, Unger RE, Otto M, Kamp G, Kirkpatrick CJ . Software-supported image quantification of angiogenesis in an in vitro culture system: application to studies of biocompatibility. Biomaterials 2002; 23: 3413–9.
Cenni E, Granchi D, Ciapetti G, Savarino L, Corradini A, Di Leo A . Cytokine expression in vitro by cultured human endothelial cells in contact with polyethylene terephthalate coated with pyrolytic carbon and collagen. J Biomed Mater Res 2000; 50: 483–9.
Cenni E, Granchi D, Ciapetti G, Cavedagna D, Corradini A, Di Leo A . Interleukin-6 expression by cultured human endothelial cells in contact with carbon coated polyethylene terephthalate. J Mater Sci Mater Med 2001; 12: 365–9.
Unger RE, Peters K, Wolf M, Motta A, Migliaresi C, Kirkpatrick CJ . Endothelialization of a non-woven silk fibroin net for use in tissue engineering: growth and gene regulation of human endothelial cells. Biomaterials 2004; 25: 5137–46.
Finkenzeller G, Arabatzis A, Geyer M, Wenger A, Bannasch H, Stark GB . Gene expression profiling reveals platelet-derived growth factor receptor alpha as a target of cell contact dependent gene regulation in an endothelial cell-osteoblast co-culture model. Tissue Eng 2006; 12: 2889–903.
Korff T, Augustin HG . Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 1998; 143: 1341–52.
Wenger A, Stahl A, Weber H, Finkenzeller G, Augustin HG, Stark GB, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng 2004; 10: 1536–47.
Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amédée J . Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 2009; 106: 390–8.
Guillotin B, Bourget C, Remy-Zolgadri M, Bareille R, Fernandez P, Conrad V, et al. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol Biochem 2004; 14: 325–32.
Guillotin B, Bareille R, Bourget C, Bordenave L, Amédée J . Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone 2008; 42: 1080–91.
Rafii S, Lyden D . Therapeutic stem and progenitor cells transplantation for organ vascularization and regeneration. Nat Med 2003; 9: 702–12.
Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S . Enhancing the vascularization of three-dimensional porous scaffolds by incorporating controlled release basic fibroblast growth factors microspheres. J Biomed Mater Res 2003; 65: 489–97.
Min JK, Kim YM, Kim YM, Kim EC, Gho YS, Kang IJ, et al. Vascular endothelial growth factor up-regulates expression of receptor activator of NF-kappa B (RANK) in endothelial cells. Concomitant increase of angiogenic responses to RANK ligand. J Biol Chem 2003; 278: 39548–57.
Richardson TP, Peters MC, Ennett AB, Mooney DJ . Polymeric system for dual growth factor delivery. Nat Biotechnol 2001; 19: 1029–34.
Orlandini M, Spreafico A, Bardelli M, Rocchigiani M, Salameh A, Nucciotti S, et al. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem 2006; 281: 17961–7.
Kleinheinz J, Stratmann U, Joos U, Wiesmann HP . VEGF-activated angiogenesis during bone regeneration. J Oral Maxillofac Surg 2005; 63: 1310–6.
Eckardt H, Bundgaard KG, Christensen KS, Lind M, Hansen ES, Hvid I . Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res 2003; 21: 335–40.
Dai J, Rabie AB . VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 2007; 86: 937–50.
Kandler B, Fischer MB, Watzek G, Gruber R . Platelet-released supernatant increases matrix metalloproteinase-2 production, migration, proliferation and tube formation of human umbilical vascular endothelial cells. J Periodontol 2004; 75: 1255–61.
Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008; 29: 4217–26.
Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002; 105: 732–8.
Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: A combined gene therapy– cell transplantation approach. Proc Natl Acad Sci USA 2008; 105: 11099–104.
Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 2008; 111: 1302–5.
Yu H, Vandevord PJ, Gong W, Wu B, Song Z, Matthew HW, et al. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res 2008; 26: 1147–52.
Acknowledgements
This study was supported by the Istituto Ortopedico Rizzoli, “Ricerca corrente” and by the Emilia-Romagna district (Italy) “Progetto di Ricerca Regione-Università: Regenerative Medicine in Osteo-articular Diseases.” The authors thank Ms. Lucy Scioscia for her help in editing English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cenni, E., Perut, F. & Baldini, N. In vitro models for the evaluation of angiogenic potential in bone engineering. Acta Pharmacol Sin 32, 21–30 (2011). https://doi.org/10.1038/aps.2010.143
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2010.143