Abstract
Prenatal invasive diagnosis of genetic conditions in the Netherlands is well organised, based on uniform indications and has a sound financial structure. Facilities for fetal karyotyping and DNA analysis are available in the 8 academic centres. Prenatal diagnosis of metabolic diseases is mainly carried out in Rotterdam. Amniocentesis, transcervical and transabdominal chorionic villus sampling are carried out in all centres, including 4 subcentres. The national Working Party on Prenatal Diagnosis started in 1985 and is a useful platform for all obstetricians and geneticists involved in prenatal diagnosis.
Similar content being viewed by others
Introduction
In this survey the situation of prenatal diagnosis (PND) of genetic conditions in the Netherlands is presented by addressing 8 basic questions which were issued by the EUCROMIC organization to all EU countries.
Firstly, some keynote figures are of importance for a better understanding of the obstetric situation in the Netherlands. The total number of births per year is about 200,000. The number of women being pregnant at the age of 36 years or older is about 15,500 (7.8%).
Facilities for PND are available in all 8 academic centres (Amsterdam-AMC, Amsterdam-AZVU, Groningen, Leiden, Maastricht, Nijmegen, Rotterdam and Utrecht). In these centres, facilities for obstetrics, clinical genetics and cytogenetic/DNA laboratories are available. Moreover there are 4 subcentres: in Arnhem, Enschede, Eindhoven and Dordrecht obstetric facilities for prenatal diagnosis are available, the samples are processed in Groningen, Utrecht, Nijmegen/Maastricht and Rotterdam, respectively. General practioners, midwives and gynaecologists refer their patients to any of these 12 centres, where pretest counselling facilities are available; for more complex genetic problems, patients are referred to the clinical geneticist. PND of metabolic diseases has been centralised in the laboratory in Rotterdam, though for some diseases other centres are involved as well. There are no private laboratories in the Netherlands.
In the context of the possible implementation of prenatal screening programmes, the organisational structure of the prenatal care in our country is relevant. The majority of the pregnant women in 1991 (57%) were primarily booked for prenatal care by midwives, while the remainder was booked by specialist obstetricians (27%) and general practitioners (16%).
Sources of Information
In the Netherlands there is no national registration of congenital anomalies and/or genetic diseases. A European Registration of Congenital Anomalies and Twins (EUROCAT) project has been started in 1981 in the north of the Netherlands. It comprises the provinces Groningen, Friesland and Drente.
A second EUROCAT registration has started in the southwestern part of the Netherlands in 1990.
Both midwives and general practitioners participate in a national obstetric registration (LVR-1), while obstetricians participate in a comparable LVR-2 registry, which started in 1982. Data on the obstetric outcome of about 80% of all pregnancies are registered. Since this registration is not focused on congenital malformations in particular, it is not surprising that of all children with malformations registered by EUROCAT only 26% were also included in the LVR.
The aforementioned centres have published (either individually or as a collaborative study) their experience with amniocentesis (AC) [1–5] with chorionic villus sampling (CVS) [6–12] and with cordocentesis [13]. Only general surveys in international journals/books are included in this article.
The national Working Party on Prenatal Diagnosis (WPD) started in 1985. This interdisciplinary working party of the Dutch Society of Obstetrics and Gynaecology and the Dutch Society of Clinical Genetics is a useful platform for all obstetricians and geneticists involved in PND. The WPD has developed and implemented quality requirements for obstetricians performing AC, CVS and cordocentesis. In these quality requirements a minimum number of procedures to be carried out annually by one obstetrician is mentioned: 30 AC and 30 CVS. In each centre a minimum of two obstetricians meeting these criteria is required. For the laboratories, quality requirements are being developed.
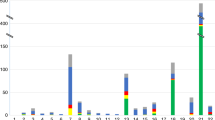
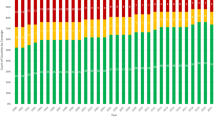
The WPD has produced detailed annual reports of all prenatal diagnostic procedures, including the follow-up of all pregnancies in the years 1989 and 1990. Provisional overall figures for the years 1991–1995 are listed in table 1.
The WPD organises scientific meetings once a year with contributions of all centres. Collaborative studies/activities under the auspices of the WPD are listed in table 2. A regular presentation is given at the ‘gynaecongres’, i.e. the national congress organised by the Dutch Society of Obstetrics and Gynaecology. In 1990, an international meeting on ‘Screening in prenatal diagnosis’ was organised in Noordwijkerhout (The Netherlands). More recently (1996) an international congress on ‘Recent advances in prenatal diagnosis for aneuploidy’ was organised in Amsterdam under the auspices of the European Down’s Syndrome Screening Group, the WPD and the Dutch Health Council.
There is no central database with the records of the pregnancies that have been tested prenatally. Such information is available only at the 8 academic centres.
Impact of Prenatal Diagnosis
We are not informed about the impact of PND on the prevalence of chromosomal disorders and severe congenital malformations. In a collaborative study carried out in 1986, it was found that an estimated prevalence at birth of Down’s syndrome was 1.06/1,000 in 1981–1982 [14]. No further national studies on the prevalence of Down’s syndrome have been carried out.
A number of studies in the Netherlands focused on the psychological impact of invasive PND [15–18]. Mainly based on these studies, standard psychosocial support was gradually introduced in all centres for women who decided to have their pregnancy terminated for genetic reasons.
Available Diagnostic Procedures
In all 8 academic centres, fetal karyotyping facilities are available.
Biochemical serum screening is a controversial issue in the Netherlands. The centre in Groningen has had a longstanding interest in prenatal screening [19–21]. However a report of the Dutch Health Council on the screening for neural tube defects [22] advised against population screening for this type of anomalies. When the triple test was introduced, our National Institute of Public Health in Bilthoven offered free testing, while the Ministry of Public Health was against mass application of this screening test. In daily practice this means that maternal serum screening is available on individual requests. In 1995, the test was carried out in 7,900 pregnancies by the laboratories in Bilthoven and Groningen. In an agreement with the health insurance companies (1995), this somewhat hybrid situation is illustrated by the following statement: ‘Maternal serum screening is not an officially recognised test for fetal Down’s syndrome. The costs of this test therefore are not paid for by the insurers. If however such a test has been carried out and the result indicates an increased risk of fetal Down’s syndrome, prenatal chromosome studies by means of amniocentesis can be carried out and costs will be reimbursed by the insurance company’.
Routine ultrasound screening is not standard practice in the Netherlands. Detailed — high resolution, real time — ultrasound investigations are targeted at women who are at increased risk of fetal structural anomalies. These include the category of women whose increased risk is based on factors known prior to the index pregnancy, e.g. a previous child with a structural anomaly for a family history of neural tube defects (type 1). The second category includes women whose increased risk is based on abnormal findings that become manifest during pregnancy (type 2). In all academic centres, facilities for high-resolution ultrasonography are available. For general surveys of the experience of individual centres, see references 23 and 24. Emotional reactions in women in late pregnancy following the ultrasound diagnosis of severe malformations were studied in 1993 [25].
The molecular-based prenatal diagnostic procedures are well organized. Because of the relatively low frequency of some conditions, a division of tasks between the 8 different laboratories has been agreed upon. A list of the conditions currently diagnosed in utero (1994) is presented in table 3.
PND of metabolic diseases has been centralised in Rotterdam. They reported about both new findings and surveys of their experience with this type of PND [see ref. 26 for a review]. In 1995, about 110 prenatal metabolic examinations were carried out. For some diseases, other centres are involved as well [27, 28].
In the Netherlands, uniform indications for PND have been developed and are listed in table 4.
Current Methods in Use for Prenatal Diagnosis
AC, transcervical and transabdominal CVS and cordocentesis are carried out in all centres. For the latter technique, the centre in Leiden has the greatest expertise: for this procedure some centres refer their patients to Leiden. The ratio between the number of AC and CVS changed from 58:42 in 1991 to 70:30 in 1995 (table 1).
Areas under Development
Fluorescence in situ labelling (FISH) on interphase nuclei and metaphase plates is available in all centres.
Projects aiming at the use of the fetal cells in the maternal circulation are in progress in Amsterdam-AMC (erythroblasts) and Leiden (erythroblasts) together with Applied Imaging. In the Amsterdam-AZVU centre, the trophoblast approach is under study.
‘First-trimester biochemical screening for Down’s syndrome’ was the title of the thesis of van Lith at the University of Groningen [29].
Screening by ultrasound for fetal chromosomal abnormalities was studied in a thesis at the University of Utrecht by Snijders [30]. In the Amsterdam-AMC centre, a research project on nuchal translucency is in progress.
Funding Arrangements for Prenatal Diagnosis
For patients with valid indications for PND, costs for obstetric and laboratory procedures are paid for by the health insurance companies, being roughly: AC/CVS: US$ 300 and chromosome/DNA studies: US$ 600, advanced ultrasound examination type 1: US$ 150 and type 2: US$ 450.
Current Legislation Surrounding Prenatal Diagnosis
A permit of the Ministry of Public Health is required for invasive PND and subsequent genetic diagnosis. Only the academic centres have such a permit. They need additional permission to collaborate with a so-called subcentre. One of the conditions for obtaining permission for PND is that the number of procedures and the number of aberrant findings are reported to the Ministry each year.
Termination of pregnancy can be carried out till 24 weeks’ gestation.
The academic centre in Maastricht has carried out research on pre-implantation diagnosis of genetic aberrations and has permission to start a diagnostic service for a limited number of indications. Their actual experience with ongoing pregnancies is still very limited.
The Population Screening Act has become effective in 1996. Permission of the Ministry of Public Health is required for the screening for incurable diseases (almost all genetic conditions). Recently, a Committee on Genetic Screening of the Dutch Health Council [31] listed criteria which must be met by genetic screening programmes prior to their implementation.
Problems and Future
With the growing number of conditions that can be diagnosed on the DNA level, policy-makers in our country have repeatedly asked for a comprehensive list of ‘severe conditions’ for which PND could be offered. The national WPD is opposed to such a list as it is neither possible, nor desirable. Last year, questions were raised about the PND of an inherited eye disease (retinitis pigmentosa) in our country. The issue was also discussed in the News section of the British Medical Journal [32]. We feel encouraged by the Dutch Minister of Public Health who defended our point of view that the future parents have an important role in the decision about the performance of PND of conditions with onset later in life.
At present, about 70% of the invasive diagnostic procedures are carried out on maternal age indication. About 55% of all women of 36 years or older actually participate in prenatal invasive diagnosis. Of the 45% of women who don’t participate, we are not informed about their possible motives. It would be important to know in what proportion of this group it is a deliberate decision of the women themselves and in what proportion of the women it is the result of insufficient information.
For termination of pregnancy after 24 weeks, professional guidelines have been developed by the Dutch Society of Obstetrics and Gynaecology. In two recent articles by the Inspectorate of Health Care in the province of North Holland, the practice was evaluated for the years 1990–1994 [33, 34]. Reasons for termination were severe fetal structural abnormalities, which in most cases were not compatible with extra-uterine life. The authors concluded that ‘gynaecologists in North Holland in general acted carefully regarding late termination of pregnancy, although they did not always observe the guidelines issued by their professional association’.
Conclusions
About 25 years have passed since the first centres in the world started with invasive procedures for PND of genetic defects. At present PND of genetic conditions in the Netherlands is well organised, based on uniform indications and has a sound financial structure. It has a solid position in the secondary prevention of a number of genetic conditions. Moreover, it has contributed to the birth of healthy children in at-risk situations, where the parents would otherwise have decided not to have children. Biochemical serum screening remains a controversial issue in the Netherlands. Optimal pretest counselling for serum screening of pregnant women, within the unique, decentralised structure of our prenatal care system, will involve large numbers of midwives, general practitioners and obstetricians, who should be well informed. This is also true when the introduction of promising new screening strategies, such as fetal nuchal translucency for Down’s syndrome and the fetal cells in maternal blood approach, is to be considered in the near future. The recently introduced Population Screening Act requires permission of the Ministry of Public Health for any genetic test before it can be introduced at the population level.
References
Niermeijer MF, Sachs ES, Jahoda M, Tichelaar-Klepper C, Kleijer WJ, Galjaard H: Prenatal diagnosis of genetic disorders. J Med Genet 1976;13:182–194.
Kleijer WJ, de Bruyn HWA, Leschot NJ: Amniotic fluid alpha-fetoprotein levels and the prenatal diagnosis of neural tube defects: A collaborative study of 2,180 pregnancies in the Netherlands. Br J Obstet Gynaecol 1978;85: 512–517.
Leschot NJ, Treffers PE, Verjaal M, der Weduwen JJ, Bennebroek Gravenhorst J, Coelingh Bennink HJT: Prenatal diagnosis of congenital malformations in 500 pregnancies. Eur J Obstet Gynecol Reprod Biol 1979;9:13–22.
Verjaal M, Leschot NJ, Treffers PE: Risk of amniocentesis and laboratory findings in a series of 1,500 prenatal diagnoses. Prenat Diagn 1981;1:173–181.
Leschot NJ, Verjaal M, Treffers PE: Risks of midtrimester amniocentesis: Evaluation in 3,000 pregnancies. Br J Obstet Gynaecol 1985; 92:804–807.
Leschot NJ, Wolf H, Verjaal M, van Prooijen-Knegt AC, de Boer EG, Kanhai HHH, Christiaens GCML: Chorionic villi sampling: Cytogenetic and clinical findings in 500 pregnancies. Br Med J 1987;295:407–410.
Sachs ES, Jahoda MGJ, Kleijer WJ, Pijpers L, Galjaard H: Impact of first trimester chromosome, DNA and metabolic studies on pregnancies at high risk. Experience with 1,000 cases. Am J Med Genet 1988;29:293–303.
Bröcker-Vriends AHJT, Briët E, Kanhai HHH, Bakker E, Dreesen JCFM, Leschot NJ, van de Kamp JJP, Pearson PL: First trimester prenatal diagnosis of haemophilia A: Two years’ experience. Prenat Diagn 1988;8:411–421.
Leschot NJ, Wolf H, van Prooijen-Knegt AC, van Asperen CJ, Verjaal M, Schuring-Blom GH, Boer K, Kanhai HHH, Christiaens GCML: Cytogenetic findings in 1,250 first trimester chorionic villi samples, with clinical follow-up of the first 1,000 pregnancies. Br J Obstet Gynaecol 1989;96:663–670.
Breed ASPM, Mantingh A, Beekhuis JR, Kloosterman MD, ten Boicher H, Anders GJPA: The predictive value of cytogenetic diagnosis after CVS. Prenat Diagn 1990; 10: 101–110.
Jahoda MGJ, Brandenburg H, Reuss A, Cohen-Overbeek TE, Wladimiroff JW, Los FJ, Sachs ES: Transcervical and transabdominal CVS for prenatal diagnosis in Rotterdam: Experience with 3,611 cases. Prenat Diagn 1991; 11:559–561.
Lunshof S, Boer K, Leschot NJ, Pomp M, Wolf H: Pregnancy outcome after transcervical CVS with flexible biopsy forceps: Evaluation of risk factors. Prenat Diagn 1995;15:809–816.
den Hollander NS, Cohen-Overbeek TE, Heydanus R, Stewart PA, Brandenburg H, Los FJ, Jahoda MG: Cordocentesis for rapid karyotyping in fetuses with congenital anomalies or severe IUGR. Eur J Obstet Gynecol Reprod Biol 1994;53:183–187.
Hustinx TWJ, Scheres JMJC, Geraedts JPM, van Elteren P: Down’s syndrome in the Netherlands (in Dutch, with English summary). Tijdschr Kindergeneeskd 1986;54:101–106.
Verjaal M, Leschot NJ, Treffers PE: Women’s experiences with second trimester prenatal diagnosis. Prenat Diagn 1982;2:195–209.
Leschot NJ, Verjaal M, Treffers PE: Therapeutic abortion on genetic indications: A detailed follow-up study of 20 patients. J Psychosom Obstet Gynaecol 1982;1:47–56.
Thomassen-Brepols LJ:Psychosocial Aspects of Prenatal Diagnosis (Dutch); thesis, Rotterdam, 1985.
Brandenburg H, de Koning W, Jahoda MGJ, Stijnen Th, de Ridder MAJ, Sachs ES, Wladimiroff JW: Reproductive behaviour and prenatal diagnosis following genetic termination of pregnancy in women of advanced maternal age. Prenat Diagn 1992;12:1031–1035.
Los FJ: Serum AFP Screening of Pregnant Women for Fetal Neural Tube Defects (Dutch); thesis, Groningen, 1980.
van Lith JMM: First trimester screening for fetal chromosomal abnormalities; preliminary results. Prenat Diagn 1991;11:621–624.
Beekhuis JR, de Wolf BTHM, Mantingh A, Heringa MP: The influence of serum screening on the amniocentesis rate in women of advanced maternal age. Prenat Diagn 1994; 14: 199–202.
Health Council of the Netherlands: Committee Neural Tube Defects. Neural Tube Defects. The Hague, Health Council, 1988, publ No 1988/15.
Buskens E, Grobbee DE, Hess J, Wladimiroff JW: Prenatal diagnosis of congenital heart disease: Prospects and problems. Eur J Obstet Gynecol Reprod Biol 1995;60:5–11.
van Zalen-Sprock RM, van Vugt JM, van Geijn HP: First and early second trimester diagnosis of anomalies of the central nervous system. J Ultrasound Med 1995;14:603–610.
Hunfeld JA, Wladimiroff JW, Passchier J, Venema-van Uden MU, Frets PG, Verhage F: Emotional reactions in women in late pregnancy following the ultrasound diagnosis of a severe or lethal malformation. Prenat Diagn 1993;13:603–612.
Kleijer WJ: Prenatal Diagnosis; in Fernandes J, Saudubray HJM, Tada K (eds): Inborn Metabolic Diseases. New York, Springer, 1990, pp 683–695.
Schutgens RBH, Wanders RJA: Peroxisomal disorders; in Holten JB (ed): The Inherited Metabolic Diseases, ed 2, Churchill Livingstone, Edinburgh, 1994, pp 243–265.
Jakobs C, ten Brink HJ, Stellaard F: Prenatal diagnosis of inherited metabolic disorders by quantitation of characteristic metabolites in amniotic fluid: Facts and future. Prenat Diagn 1990;10:265–271.
van Lith J: First Trimester Biochemical Screening for Down’s Syndrome; thesis, Groningen. Amsterdam, Rodopi, 1994.
Snijders RJM: Screening by Ultrasound for Fetal Chromosomal Abnormalities; thesis, Utrecht. Utrecht, Elinkwijk, 1993.
Health Council of the Netherlands: Committee Genetic Screening. Genetic Screening. The Hague, Health Council, 1994, publ No 1994/22E.
Sheldon T: Prenatal testing leads to row in the Netherlands. Br Med J 1995;311:1187.
van der Wal G, Bosma JM, Hosman-Benjaminse SL: Late termination of pregnancy in North Holland. I. Incidence and abnormalities (in Dutch, with English summary). Ned Tijdschr Geneeskd 1996;140:600–604.
Bosma JM, van der Wal G, Hosman-Benjaminse SL: Late termination of pregnancy in North Holland. II. Carefulness before and review afterwards (in Dutch, with English summary). Ned Tijdschr Geneeskd 1996; 140:605–609.
Acknowledgments
We thank Dr. H.I.J. Wildschut, Prof. Dr. F.A. Beemer, Dr. T. Hasaart and Dr. I. van Kamp for their comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Leschot, N.J., Kloosterman, M.D. & Dutch Working Party of Prenatal Diagnosis. Prenatal Diagnosis in the Netherlands. Eur J Hum Genet 5 (Suppl 1), 51–56 (1997). https://doi.org/10.1007/BF03405962
Issue Date:
DOI: https://doi.org/10.1007/BF03405962