Abstract
Hirschsprung disease (HSCR, aganglionic megacolon) is a frequent congenital malformation regarded as a multigenic neurocristopathy. Four susceptibility genes have recently been identified in HSCR, namely the RET proto-oncogene, the glial cell line-derived neurotrophic factor (GDNF), the endothelin B receptor (EDNRB) and the endothelin-3 genes (EDN3). Homozygosity for EDN3 mutations has been previously shown to cause the Shah-Waardenburg syndrome, a combination of HSCR with features of the Waardenburg syndrome. Here, we report on heterozygous EDN3 missense mutations in isolated HSCR. The present data give further support to the role of the endothelin-signaling pathway in the development of neural crest-derived enteric neurons. They also suggest the possibility that either recessive or weakly penetrant dominant alleles could occur at the EDN3 locus, depending on the nature of the mutation.
Similar content being viewed by others
Introduction
Hirschsprung disease (HSCR, aganglionic megacolon) is a common congenital malformation (1/5,000 live births) characterized by the absence of intrinsic ganglion cells in the myenteric and submucosal plexuses along variable lengths of the gastrointestinal tract [1]. Since enteric neurons are derived from the vagal neural crest, HSCR is regarded as a neurocristopathy. The disease results in intestinal obstruction in neonates and severe constipation in infants and adults [2, 3]. Although more than 80% of HSCR cases are sporadic, several pedigrees have been described and a body of clinical, genetic and molecular data support the genetic heterogeneity of HSCR. In 1994, the RET proto-oncogene on chromosome 10 has been recognized as a major disease-causing gene in HSCR [4–7]. Yet, RET mutations only account for 50 and 15–20% of familial and sporadic cases of HSCR, respectively [8]. Recently, homozygous mutations of the endothelin-3 (EDN3) gene on chromosome 20 have been identified in consanguineous HSCR families harboring other malformations of neural crest-derived cells, namely pigmentary anomalies and deafness [Shah-Waardenburg syndrome, 9, 10]. However, the question of whether EDN3 mutations could also account for isolated HSCR remained unanswered. Here, we report a homozygous EDN3 mutation in a family with the Shah-Waardenburg syndrome (WS) and two heterozygous missense EDN3 mutations in patients with isolated HSCR. These data further support the role of the endothelin signaling pathway in determining HSCR and raise the question of the mechanism(s) through which EDN3 gene mutations could be deleterious.
Patients and Methods
A total of 174 probands with isolated HSCR (59 families, 117 sporadic cases), and 6 patients with the WS-HSCR association (2 families, including 1 initially described by Van Camp et al. [11], and 4 sporadic cases) were screened for mutations in the coding sequence of the EDN3 gene. Histopathological criteria for HSCR were (1) absence of enteric plexuses with histological evaluation of the aganglionic tract, and (2) increased acetylcholinesterase histochemical staining in nerve fibers. Long- and short-segment HSCR were defined according to the length of the aganglionic segment compared to the rectosigmoidal flexure. DNA was isolated from peripheral blood lymphocytes by standard methods. For PCR amplification of the EDN3 coding sequence, 6 oligonucleotide primer pairs, encompassing the 5 exons and flanking intronic sequences, were used. Oligonucleotide sequences were as follows: (forward/reverse, 5′-3′) exon 1, CAA GCG GCC GTC CTC CTG GTC CGG T/CTT CTC CGC GCC TCG GTC C (180 bp); exon 2A, CCC TCC TCA GGT GTT TGG G/TCG GCC GCC TGC TCC TGC (239 bp); exon 2B, TGG CGA GGA GAC TGT GGC T/GAA TGA GCA GAT GTG GGC G (218 bp); exon 3, GCT CAC CTA ACA TTA CCC/CCG AGG GTT GAT GCA TAT (259 bp); exon 4, GGT GGG GAA GAG GAA GTC A/CCC GAA GGA ATG ACA CGT C (200 bp); exon 5, TCA CAG AAC TAC AGA GCT AC/ACT GTG TGT GAG CAA TGA CA. Thirty-two amplification cycles were performed with the following annealing temperatures: 65°C, 60°C (6% formamide), 60°C, 52°C, 65°C and 55°C for exons 1, 2A, 2B, 3, 4, 5, respectively. For single-strand conformation polymorphism analysis (SSCP), PCR products were heated (10 min at 94°C), loaded onto a Hydrolink MDE gel (Bioprobe), then electrophoresed and autoradiographed for 36 h. In case of SSCP mobility shifts, amplified genomic DNA was sequenced on both strands by the fluorometric method (Dye Deoxy Terminator Cycle Sequencing kit, Applied Biosystems). For each DNA variant, 100 normal controls (200 chromosomes) were screened in addition to the unaffected family members in order to distinguish nonpathological base substitutions from disease-causing mutations.
Results
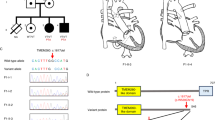
Our strategy was to analyze the coding sequence of the EDN3 gene for mutations by using a combination of SSCP analysis and direct DNA sequencing. We found abnormal SSCP migration patterns in 3 unrelated HSCR probands (table 1). A homozygous G to T transversion at the first nucleotide of codon 55 was detected in exon 2 of the EDN3 gene in a patient with the WS-HSCR association (fig. 1). This base substitution is a nonsense mutation (E55X) located upstream from the mature EDN3 sequence, which presumably results in the complete absence of EDN3 in the proband. Both E55X mutant alleles were inherited from the consanguineous parents who were first cousins (f = 1/16) and did not show any clinical sign of WS and HSCR. None of the sibs carried the mutation, but an older brother had died with long segment HSCR and WS features [11] (fig. 1A). In 3 sporadic cases with WS-HSCR, no mutation was found in the open reading frame of EDN3.
In 2 patients with isolated and sporadic HSCR, direct DNA sequence showed heterozygosity for a G to A transition in exons 1 and 5 of the EDN3 gene, respectively, at codons 17 and 224 (fig. 2). Both missense mutations (A17T and A224T) are predicted to result in the replacement of an alanine by a threonine residue in the preproen-dothelin. In particular, the A17T mutation is located in the peptide signal sequence. In both cases, the mutation was inherited from the asymptomatic mother.
A Pedigree of the S120 family. Direct DNA sequence of exon 1 of the EDN3 gene showing the A17T mutation. The G to A transversion is indicated by an arrow. B Pedigree of the S65 family. Direct DNA sequence of exon 5 of the EDN3 gene showing the A224T mutation. The G to A transition is indicated by an arrow. + = Normal allele; m = mutant allele.
Each of the three mutations was absent in 100 unrelated healthy individuals (200 chromosomes, p < 0.01). Finally, the rest of the EDN3 coding sequence was normal, and no abnormal SSCP pattern was detected in the coding sequences of the RET, GDNF and EDNRB genes (data not shown).
Discussion
The EDN3 gene encodes a large inactive preproendo-thelin-3 precursor which yields a biologically active 21-amino acid peptide after proteolytic cleavages at furin and endothelin-converting enzyme 1 cleavage sites, respectively. EDN3 belongs to a superfamily that also includes EDN1 and EDN2. Endothelins exert their biological effects through the heterotrimeric G protein-coupled heptahelical receptors A (EDNRA) and B (EDNRB) [12, 13]. Recently, transgenic mice with a homozygous knock-out mutation of Edn3 were shown to have megacolon and coat color spotting (mutant lethal spotted s′[14, 15]. This gives support to the view that Edn3 is involved in the development of the enteric nervous system as previously shown for its receptor, Ednrb. Moreover, homozygous mutations of the human EDN3 gene were shown to be associated with the pleiotropic features observed in the WS-HSCR syndrome in human [9, 10]. Here, we report the existence of a homozygous nonsense EDN3 mutation (E55X) in another family with the WS-HSCR association. Since the E55X mutation is located upstream from the mature EDN3 coding sequence, the nonsense mutation is predicted to result in the complete absence of the active form of the peptide in patient II1 (fig. 1). Both healthy parents were found to be heterozygous, suggesting that the E55X mutation might behave as a recessive allele.
However, the question of whether EDN3 mutations could also account for isolated HSCR remained unanswered. Here we report on two heterozygous base substitutions of the EDN3 gene in a large series of nonsyndromic HSCR patients (174 probands). The missense mutations reported here occurred in conserved regions of the protein. In particular, the A17T mutation is located in the signal sequence of the preproendothelin that is critical for the correct addressing of the protein. Conversely, the A224T mutation lies in a region that is enzymatically spliced off during the processing of the preproendothelin. However, the substitution of a threonine for an alanine is a change from a nonpolar to a polar amino acid that might well affect the proper enzymatic cleavage of preproendothelin, a process in which the A17T mutation might also be involved. Thus, further in vitro functional studies are necessary to demonstrate the pathogenicity of the A17T and A224T mutations, although their absence in 200 control chromosomes (p < 0.01) argues against neutral polymorphisms which cannot be excluded, and suggests that they might be significant in the disease phenotype. Interestingly, both EDN3 mutations reported here were consistently inherited from an unaffected mother carrier. This feature suggests a low penetrance of the trait and, eventually, the existence of one or more modifier loci in HSCR individuals carrying heterozygote EDN3 mutations. Alternately, genomic imprinting of the EDN3 locus with a preferentially expressed maternal allele could be discussed.
Thus, it is possible that the dosage of EDN3 mutations could have various consequences on the resulting phenotype: heterozygosity would predispose to isolated HSCR with incomplete penetrance, while homozygosity would result in more complex neurocristopathies associating HSCR and WS features. Yet, it is worth remembering that mutations of either the EDN3 [9, this report] or the EDNRB [16] genes were found in only 3/6 WS-HSCR probands examined so far in our series (2 familial forms including the one reported here, and 1 sporadic case, table 1), suggesting that other gene(s) could be involved in this complex neurocristopathy [9, 10, 16–18].
In conclusion, we propose here that the EDN3 gene could be a rare susceptibility locus in nonsyndromic HSCR (2/174 probands in our series) as recently shown for another rare neurocristopathy, namely the congenital central hypoventilation syndrome [19]. Hitherto, four predisposing genes have been identified in isolated and syndromic HSCR, namely RET, GDNF, EDNRB, and EDN3 [6, 7, 9, 10, 16–18, 20, 21]. This is in agreement with the prediction of genetic heterogeneity in HSCR. Moreover, the fact that GDNF mutations have been found so far in the context of mutations at the RET locus gives also support to the multigenic mode of inheritance of HSCR [20, 21]. Finally, while little is knownregarding the physiological role of EDN3, the involvement of EDN3 in isolated HSCR suggests that both RET and EDNRB signaling pathways are crucial for the development of the enteric nervous system. Further studies will hopefully address the question of whether mutations in genes encoding cytoplasmic proteins involved in signal transduction of the tyrosine kinase and the G-protein-coupled pathways could also be involved in the HSCR phenotype especially as cross talks between those two pathways have been demonstrated [21].
References
Okamoto E, Ueda T: Embryogenesis of intramural ganglia of the gut and its relation to Hirschsprung’s disease. J Pediatr Surg 1967;2: 437–443.
Polley TZ, Coran AG: Hirschsprung’s disease in the newborn. Pediatr Surg Int 1986; 1:80–83.
Lesser PB, El-Nahas AM, Luke P: Adult-onset Hirschsprung’s disease. JAMA 1979;242:742–752.
Lyonnet S, Bolino A, Pelet A, Abel L, Nihoul-Fékété C, Briard ML, Mok-Siu V, Kääriainen H, Martucciello G, Lerone M, Puliti A, Luo Y, Weissenbach J, Devoto M, Munnich A, Romeo G: A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nature Genet 1993;4:346–350.
Angrist M, Kauffman E, Slaugenhaupt SA, Matise TC, Puffenberg EG, Washington SS, Lipson A, Cass DT, Reyna T, Weeks DE, Sieber W, Chakravarti A: A gene for Hirschsprung disease (megacolon) in the pericentromeric region of human chromosome 10. Nature Genet 1993;4:351–356.
Romeo G, Ronchetto P, Luo Y, Arone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kääriainen H, Martucciello G: Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature 1994;367:377–378.
Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fékété C, Ponder BAJ, Munnich A: Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature 1994;367:378–380.
Attié T, Pelet A, Edery P, Eng C, Mulligan LM, Amiel J, Boutrand L, Beldjord C, Nihoul-Fékété C, Munnich A, Ponder BAJ, Lyonnet S: Diversity of RET mutations in Hirschsprung disease. Hum Mol Genet 1995;4:1381–1386.
Edery P, Attié T, Amiel J, Pelet A, Eng C, Hofstra RMW, Bidaud C, Munnich A, Lyonnet S: Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease. Nature Genet 1996;12:442–444.
Hofstra R, Osing J, Tan-Sindhunata G, Wu Y, Kamsteeg E, Stulp R, Ravenswaaij-Arts C, Majoor-Krakauer D, Angrist M, Chakravarti A, Meijers C, Buys C: A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nature Genet 1996;12:445–447.
Van Camp G, Van Thienen MN, Handing I, Van Roy B, Milunsky A, Read AP, Baldwin CT, Farrer LA, Bonduelle M, Standaert L, Meire F, Willems PJ: Chromosome 13q deletion with Waardenburg syndrome: Further evidence for a gene involved in neural crest function on 13q. J Med Genet 1995;32:531–536.
Ogawa Y, Nakao K, Arai H, Nakawaga O, Hosoda K, Suga SI, Nakanishi S, Imura H: Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biophys Biochem Res Commun 1991;178:248–255.
Sakurai T, Yanagisawa M, Masakai T: Molecular characterization of endothelin receptors. Trends Pharmacol Sci 1992;13:103–108.
Hosada K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M: Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color mice. Cell 1994;79:1267–1276.
Greenstein Baynash A, Hosada K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M: Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 1994;79:1277–1285.
Puffenberger EG, Hosoda K, Washington SS, Nakao K, deWit D, Yanagisawa N, Chakravarti A: A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell 1994;79:1257–1266.
Attié A, Till M, Pelet A, Amiel J, Edery P, Boutrand L, Munnich A, Lyonnet S: Mutation of the endothelin-receptor B gene in the Waardenburg-Hirschsprung disease. Hum Mol Genet 1995;4:2407–2409.
Amiel J, Attié T, Jan D, Pelet A, Edery P, Bidaud C, Lacombe D, Tam P, Simeoni J, Flori E, Fékété N, Munnich A, Lyonnet S: Heterozygous endothelin receptor B mutations in isolated Hirschsprung disease. Hum Mol Genet 1996;5:355–357.
Bolk S, Angrist M, Xie J, Yanagisawa M, Silvestri JM, Chakravarti A: Endothelin-3 frame-shift mutation in congenital central hypoventilation syndrome. Nature Genet 1996; 13:395–396.
Salomon R, Attié T, Pelet A, Bidaud C, Eng C, Nihoul-Fékété C, Goulet O, Sarnaki S, Ricour C, Munnich A, Lyonnet S: Germline mutations of the RET ligand, GDNF, are not sufficient to cause Hirschsprung disease. Nature Genet 1996;14:345–347.
Angrist M, Bolk S, Halushka M, Lapchak PA, Chakravarti A: Germline mutations in glial cell-line derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nature Genet 1996;14:341–343.
Daub H, Weiss F, Wallasch C, Ullrich A: Role of transactivation of the EGF receptor in signaling by G-protein-coupled receptor. Nature 1996;379:557–560.
Acknowledgements
We thank the HSCR families for their cooperation, and the many surgeons, pediatricians and geneticists who referred their patients to us. This study was supported by the Groupement de Recherche et d’Etudes des Génomes (GREG), the Caisse Nationale d’Assurance Maladie des travailleurs indépendants (CANAM), the Association pour la Recherche contre le Cancer (ARC), and the Assistance Publique-Hôpitaux de Paris (PHRC-94). R.S. is the recipient of fellowships from the CANAM-AP and the Guigoz Foundation. J.A. and T.A. are recipients of fellowships from the ARC and the Fondation pour la Recherche Médicale (FRM), respectively.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bidaud, C., Salomon, R., Van Camp, G. et al. Endothelin-3 Gene Mutations in Isolated and Syndromic Hirschsprung Disease. Eur J Hum Genet 5, 247–251 (1997). https://doi.org/10.1007/BF03405925
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03405925