Abstract
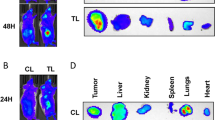
A nonviral gene carrier, calcium carbonate (CaCO3) nanoparticle, was evaluated for efficient in vitro and in vivo delivery of small interfering RNA (siRNA) targeting vascular endothelial growth factor-C (VEGF-C). The chemically synthesized CaCO3 nanoparticle has a 58 nm diameter and +28.6 mV positive surface charge. It is capable of forming a CaCO3 nanoparticle–DNA complex and transferring DNA into targeted cells with high transfection efficiency while effectively protecting the encapsulated DNA from degradation. Furthermore, the CaCO3 nanoparticle–DNA complex has no obvious cytotoxicity for SGC-7901 cells, while a liposome–DNA complex exhibited measurable cytotoxicity. SGC-7901 cells transfected with a VEGF-C-targeted siRNA via CaCO3 nanoparticle exhibit significantly reduced VEGF-C expression as measured by real-time PCR and enzyme-linked immunosorbent assay; whereas no decrease in VEGF-C expression is observed in cells treated by control transfection. Transfection of SGC-7901 cells with VEGF-C siRNA via CaCO3 nanoparticle also dramatically suppresses tumor lymphangiogenesis, tumor growth and regional lymph-node metastasis in subcutaneous xenografts. Significant downregulation of VEGF-C messenger RNA expression in a subcutaneous xenograft derived from VEGF-C siRNA-treated SGC-7901 cells was confirmed by real-time PCR as compared to controls. We conclude that CaCO3 nanoparticle is a novel and nonviral system for effective delivery of siRNA for cancer gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ . Cancer statistics. CA Cancer J Clin 2003; 53: 5–26.
Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–191.
Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001; 20: 672–682.
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7: 151–152.
Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999; 5: 1823–1829.
Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 1999; 80: 309–313.
Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer 2000; 83: 887–891.
Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S . Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res 2000; 6: 2431–2439.
Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL et al. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res 2000; 6: 4278–4286.
Kajita T, Ohta Y, Kimura K, Tamura M, Tanaka Y, Tsunezuka Y et al. The expression of vascular endothelial growth factor C and its receptors in non-small cell lung cancer. Br J Cancer 2001; 85: 255–260.
Hashimoto I, Kodama J, Seki N, Hongo A, Yoshinouchi M, Okuda H et al. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer 2001; 85: 93–97.
Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K et al. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer 2001; 93: 662–666.
McManus MT, Sharp PA . Gene silencing in mammals by small interfering. Nat Rev Genet 2002; 3: 737–747.
Elbashir SM, Harborth J, Weber K, Tuschl T . Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 2002; 26: 199–213.
Hannon GJ . RNA interference. Nature 2002; 418: 244–251.
Shi Y . Mammalian RNAi for the masses. Trends Genet 2003; 19: 9–12.
Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK et al. siRNA-directed inhibition of HIV-1 infection. Nat Med 2002; 8: 681–686.
Brummelkamp TR, Bernards R, Agami R . Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2002; 2: 243–247.
Blaesé M, Blankenstein T, Brenner M, Cohen-Haguenauer O, Gansbacher B, Russeu S et al. Vectors in cancer therapy: how will they deliver? Cancer Gene Ther 1995; 2: 291–297.
Yang Y, Nunes FA, Berenncsi K, Furth EE, Gonczol E, Wilson JM . Cellular immunity to viral antigens limits E1-deleted adenovirus for gene therapy. Proc Natl Acad Sci USA 1994; 91: 4407–4411.
Engelhardt JE, Ye X, Doranz B, Wilson JM . Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA 1994; 91: 6196–6200.
Tenenbaum L, Lehtonen E, Monahan PE . Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther 2003; 3: 545–565.
Han S, Mahato RI, Sung YK, Kin SW . Development of biomaterials for gene therapy. Mol Ther 2000; 2: 302–317.
Ferrara N . Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 1999; 237: 1–30.
Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001; 20: 672–682.
Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002; 296: 1811–1812.
Kaipanien A, Korhonen J, Mustonen T, Van Hinsberqh VW, Fang GH, Dumont D et al. Expression of the fms-like tyrosine kinase 4 gene become restricted to lymphatic endothelium during development. Proc Natl Sci USA 1995; 92: 3566–3570.
Li K, Lin SY, Brunicardi FC, Seu P . Use of RNA interference to target cyclin E-overexpressing hepatocellular carcinoma. Cancer Res 2003; 63: 3593–3597.
Wu H, Hait WN, Yang JM . Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res 2003; 63: 1515–1519.
Wannenes F, Ciafre SA, Niola F, Frajese G, Farace MG . Vector-based RNA interference against vascular endothelial growth factor-A significantly limits vascularization and growth of prostate cancer in vivo. Cancer Gene Ther 2005; 12: 926–934.
Lapteva N, Yang AG, Sanders DE, Strube DE, Chen SY . CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther 2004; 12: 84–89.
Stege A, Priebsch A, Nieth C, Lage H . Stable and complete overcoming of MDR1/p-glycoprotein-mediated multidrug resistance in human gastric carcinoma cells by RNA interference. Cancer Gene Ther 2004; 11: 696–700.
Xu XM, Wang D, Shen Q, Chen YQ, Wang MH . RNA-mediated gene silencing of the RON receptor tyrosine kinase alters oncogenic phenotypes of human colorectal carcinoma cells. Oncogene 2004; 23: 8464–8474.
Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS . RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene 2004; 23: 8486–8496.
Bigger I, Dubernet C, Couvreur P . Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 2002; 54: 631–651.
Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ . Pol(lactic acid)-poly(ethylene glycol) nanoparticles as new carries for the delivery of plasmid DNA. Control Release 2001; 75: 211–224.
Liu T, Tang AF, Zhang Gy, Yu Xiang C, Zhang JY, Shu Song P et al. Calcium phosphate nanoparticles as a novel nonviral vector efficiently transfection of DNA in cancer gene therapy. Cancer Biother Radiopharm 2005; 20: 141–149.
Truong-Le VL, Walsh SM, Schweibert E, Mao HQ, Guggino WB, August JT et al. Gene transfer by DNA-gelatin nanospheres. Arch Biochem Biophys 1999; 361: 47–56.
Cohen Y, Levv RJ, Gao J, Fishbein I, Kousae V, Sosnowski S et al. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Therapy 2007; 7: 1896–1905.
Maitra A . Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev Mol Diagn 2005; 5: 893–905.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Xw., Liu, T., Chen, Yx. et al. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther 15, 193–202 (2008). https://doi.org/10.1038/sj.cgt.7701122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701122
Keywords
This article is cited by
-
Multifunctional nanocarriers for targeted drug delivery and diagnostic applications of lymph nodes metastasis: a review of recent trends and future perspectives
Journal of Nanobiotechnology (2023)
-
Hybrid Lymphatic Drug Delivery Vehicles as a New Avenue for Targeted Therapy: Lymphatic Trafficking, Applications, Challenges, and Future Horizons
The Journal of Membrane Biology (2023)
-
KDM6B promotes gastric carcinogenesis and metastasis via upregulation of CXCR4 expression
Cell Death & Disease (2022)
-
Molecular targeted treatment and drug delivery system for gastric cancer
Journal of Cancer Research and Clinical Oncology (2021)
-
MSI2-TGF-β/TGF-β R1/SMAD3 positive feedback regulation in glioblastoma
Cancer Chemotherapy and Pharmacology (2019)