Abstract
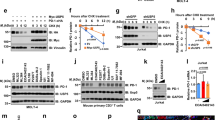
Steady-state protein levels are determined by the balance between protein synthesis and degradation. Protein half-lives are determined primarily by degradation, and the major degradation pathways involve either lysosomal destruction or an ATP-dependent process involving ubiquitination to target proteins to the proteosome. Studies have shown that multiple tumor-suppressor proteins are ubiquitinated and degraded by the 26S proteasome. In the present study, we investigated whether the tumor suppressor/cytokine melanoma differentiation-associated gene-7/interleukin-24 gene (MDA-7/IL-24) protein is ubiquitinated and its degradation controlled by the proteasome. Treatment of ovarian (2008) and lung (H1299) tumor cells with adenoviral delivery of mda-7 (Ad-mda7) or Ad-mda7 plus the proteosome inhibitor MG132 showed that MDA-7 protein expression was dependent upon proteosome activity. Western blot and immunoprecipitation analyses verified that the MDA-7 protein was ubiquitinated and that ubiquitinated-MDA-7 levels were increased in MG132-treated cells. These results were confirmed using small interfering RNA (siRNA)-mediated knockdown of ubiquitin. Furthermore, ubiquitinated MDA-7 protein was degraded by the 26S proteasome, as MDA-7 accumulation was observed only when cells were treated with MG132 but not with lysosome or protease inhibitors. Inhibition of the catalytic β-5 subunit of the 20S proteasome using siRNA resulted in MDA-7 protein accumulation. Finally, treatment of tumor cells with Ad-mda7 plus the proteasome inhibitor bortezomib resulted in increased tumor cell killing. Our results show that MDA-7/IL-24 is ubiquitinated and degraded by the 26S proteasome. Furthermore, inhibition of MDA-7 degradation results in enhanced tumor killing, identifying a novel anticancer strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walsh G, Jefferis R . Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 2006; 24: 1241–1245.
Yang XJ, Gregoire S . A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol Cell 2006; 23: 779–786.
Dahlmann B . Proteasomes. Essays Biochem 2005; 41: 31–48.
Reinstein E, Ciechanover A . Narrative review: protein degradation and human diseases: the ubiquitin connection. Ann Intern Med 2006; 145: 676–684.
Hirano Y, Ronai Z . A new function for p53 ubiquitination. Cell 2006; 127: 675–677.
Yamasaki S, Yagishita N, Sasaki T . Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase ‘Synoviolin’. EMBO J 2007; 26: 113–122.
Trotman LC, Wang X, Alimonti Z . Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 2007; 128: 141–156.
den Besten W, Kuo ML, Tago K, Williams RT, Sherr CJ . Ubiquitination of, and sumoylation by, the Arf tumor suppressor. Isr Med Assoc J 2006; 8: 249–251.
Ying H, Xiao ZX . Targeting retinoblastoma protein for degradation by proteasomes. Cell Cycle 2006; 5: 506–508.
Brignole C, Marimpietri D, Pastorino F, Nico B, Di Paolo D, Cioni M et al. Effect of bortezomib on human neuroblastoma cell growth, apoptosis, and angiogenesis. Natl Cancer Inst 2006; 98: 1142–1157.
Roccaro AM, Hideshima T, Richardson PG, Russo D, Ribatti D, Vacca A et al. Bortezomib as an antitumor agent. Curr Pharm Biotechnol 2006; 7: 441–448.
Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW et al. MDA-7/IL-24 is a unique cytokine–tumor suppressor in the IL-10 family. Int Immunopharmacol 2004; 4: 649–667.
Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther 2003; 2: S23–S37.
Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F et al. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med 2001; 7: 271–282.
Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R . Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Therapy 2000; 7: 2051–2057.
Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H et al. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer Res 2002; 62: 2239–2243.
Gopalan B, Litvak A, Sharma C, Mhashilkar AM, Chada S, Ramesh R . Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Res 2005; 65: 3017–3024.
Pataer A, Bocangel D, Chada S, Roth JA, Hunt KK, Swisher SG . Enhancement of adenoviral MDA-7-mediated cell killing in human lung cancer cells by geldanamycin and its 17-allyl- amino-17-demethoxy analogue. Cancer Gene Ther 2007; 14: 2–18.
Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE . Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther 2004; 9: 818–828.
Shanker M, Gopalan B, Patel S, Bocangel D, Chada S, Ramesh R . Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways. Cancer Lett 2007; 254: 217–226.
Oida Y, Gopalan B, Miyahara R, Inoue S, Branch CD, Mhashilkar AM et al. Sulindac enhances adenoviral vector expressing mda-7/IL-24-mediated apoptosis in human lung cancer. Mol Cancer Ther 2005; 4: 291–304.
Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC et al. mda-7 in combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol Ther 2007; 15: 287–294.
Ramesh R, Ito I, Saito Y, Wu Z, Mhashikar AM, Wilson DR et al. Local and systemic inhibition of lung tumor growth after nanoparticle-mediated mda-7/IL-24 gene delivery. DNA Cell Biol 2004; 23: 850–857.
Tahara I, Miyake K, Hanawa H, Kurai T, Hirai Y, Ishizaki M et al. Systemic cancer gene therapy using adeno-associated virus type 1 vector expressing MDA-7/IL24. Mol Ther 2007 [E-pub ahead of print].
Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res 2003; 63: 5105–5113.
Ward CL, Omura S, Kopito RR . Degradation of CFTR by the ubiquitin–proteasome pathway. Cell 1995; 83: 121–127.
Murata S . Multiple chaperone-assisted formation of mammalian 20S proteasomes. IUBMB Life 2006; 58: 344–348.
Zhu H, Guo W, Zhang L, Wu S, Teraishi F, Davis JJ et al. Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther 2005; 4: 781–786.
Takigawa N, Vaziri SA, Grabowski DR, Chikamori K, Rybicki LR, Bukowski RM et al. Proteasome inhibition with bortezomib enhances activity of topoisomerase I-targeting drugs by NF-kappaB-independent mechanisms. Anticancer Res 2006; 26: 1869–1876.
Acknowledgements
We thank Debbie Smith for manuscript preparation, Michael Worley and the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance. This study was supported by funds from the National Cancer Institute grants RO1-CA 102716 and PO1 CA 06294 (R Ramesh) and CA 89778, CA 88421 and CA 097598 (S Chada); Cancer Center Support grant CA 16672 and a sponsored research agreement with Introgen Therapeutics, Inc. We like to disclose financial interest; RR is a consultant for Introgen Therapeutics and SC is an employee of Introgen Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopalan, B., Shanker, M., Scott, A. et al. MDA-7/IL-24, a novel tumor suppressor/cytokine is ubiquitinated and regulated by the ubiquitin–proteasome system, and inhibition of MDA-7/IL-24 degradation enhances the antitumor activity. Cancer Gene Ther 15, 1–8 (2008). https://doi.org/10.1038/sj.cgt.7701095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701095
Keywords
This article is cited by
-
Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities
Journal of Molecular Signaling (2013)
-
Heat-shock protein 90 inhibitors synergistically enhance melanoma differentiation-associated gene-7-mediated cell killing of human pancreatic carcinoma
Cancer Gene Therapy (2013)
-
MicroRNA-205 Directly Regulates the Tumor Suppressor, Interleukin-24, in Human KB Oral Cancer Cells
Molecules and Cells (2013)