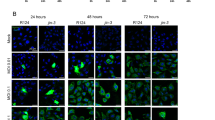
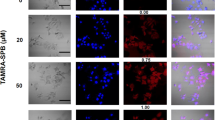
Abstract
The aim of the present study was to determine whether a prostate-specific amplicon, containing a probasin-derived promoter (ARR2PB) upstream of an essential Herpes simplex virus-1 (HSV-1) viral gene, infected-cell polypeptide 4 (ICP4), could complement an HSV-1 helper virus with this gene deleted (ICP4−) and cause lytic replication specifically in prostate cancer cells. Two amplicon constructs, CMV-ICP4 and ARR2PB-ICP4, were packaged by a replication-deficient ICP4− helper virus. The amplicon viruses could complement ICP4− helper viruses to efficiently replicate and cause cell lysis in prostate cancer cells. Intratumoral injection of LNCaP human prostate cancer xenografts with either amplicon/helper virus resulted in >75% reduction in tumor volume and serum prostate specific antigen (PSA). Histological and Q-PCR (quantitative PCR) analyses indicated that the toxicity in nontumor tissues was much lower with ARR2PB-ICP4 than with CMV-ICP4 amplicon/helper virus. In conclusion, a replication-deficient HSV-1 virus could be complemented by an amplicon virus to restore its oncolytic activity in a tissue-specific and low toxicity fashion, illustrating that this approach could be a potentially useful strategy for developing an oncolytic viral therapy for prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Edwards BK, Brown LM, Wingo PA, Howe HL, Ward E, Ries LAG et al. Annual Report to the Nation on the Status of Cancer, 1975–2002, Featuring Population-Based Trends in Cancer Treatment. J Natl Cancer Inst 2005; 97: 1407–1414.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–130.
Bagnall S, Klotz L . Conservative versus radical therapy of prostate cancer: how have recent advances in molecular markers and imaging enhanced our ability to prognosticate risk? Semin Oncol 2003; 30: 587–595.
Fleshner N . Defining high-risk prostate cancer: current status. Can J Urol 2005; 12: 14–17; discussion 94–96.
Oefelein MG, Agarwal PK, Resnick MI . Survival of patients with hormone refractory prostate cancer in the prostate specific antigen era. J Urol 2004; 171: 1525–1528.
Gleave M, Goldenberg SL, Bruchovsky N, Rennie P . Intermittent androgen suppression for prostate cancer: rationale and clinical experience. Prostate Cancer Prostatic Dis 1998; 1: 289–296.
Albertsen PC, Hanley JA, Fine J . 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005; 293: 2095–2101.
Martel CL, Gumerlock PH, Meyers FJ, Lara PN . Current strategies in the management of hormone refractory prostate cancer. Cancer Treat Rev 2003; 29: 171–187.
Kent EC, Hussain MH . The patient with hormone-refractory prostate cancer: determining who, when, and how to treat. Urology 2003; 62: 134–140.
So A, Gleave M, Hurtado-Col A, Nelson C . Mechanisms of the development of androgen independence in prostate cancer. World J Urol 2005; 23: 1–9.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Petrylak DP . Future directions in the treatment of androgen-independent prostate cancer. Urology 2005; 65: 8–12.
Satoh T, Irie A, Egawa S, Baba S . In situ gene therapy for prostate cancer. Curr Gene Ther 2005; 5: 111–119.
Hsieh CL, Kubo H, Chung LW . Gene therapy for prostate cancer bone metastasis. Gene therapy targeting bone metastasis. Cancer Treat Res 2004; 118: 231–290.
Foley R, Lawler M, Hollywood D . Gene-based therapy in prostate cancer. Lancet Oncol 2004; 5: 469–479.
Lu Y . Viral based gene therapy for prostate cancer. Curr Gene Ther 2001; 1: 183–200.
Lachmann R . Herpes simplex virus-based vectors. Int J Exp Pathol 2004; 85: 177–190.
Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL . Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med 1995; 1: 938–943.
Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy 2000; 7: 867–874.
MacKie RM, Stewart B, Brown SM . Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet 2001; 357: 525–526.
Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy 2003; 10: 292–303.
Coffin RS, Liu B, Han Z, Simpson G, Hu J, Coombes C et al. OncoVEX: A family of oncolytic herpes simplex viruses optimized for therapeutic use. ASCO Annual Meeting: Orlando, FL. 2004.
Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207). FASEB J 1999; 13: 1325–1334.
Roizman B, Knipe DM . Herpes Simplex Viruses and Their Replication. Lippincott Williams & Wilkins: Philadelphia, PA, 2001.
Mocarski ES, Roizman B . Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell 1982; 31: 89–97.
Zhang J, Thomas TZ, Kasper S, Matusik RJ . A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology 2000; 141: 4698–4710.
Johnson PA, Miyanohara A, Levine F, Cahill T, Friedmann T . Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol 1992; 66: 2952–2965.
Spaete RR, Frenkel N . The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell 1982; 30: 295–304.
Wei C, Willis RA, Tilton BR, Looney RJ, Lord EM, Barth RK et al. Tissue-specific expression of the human prostate-specific antigen gene in transgenic mice: Implications for tolerance and immunotherapy. Proc Natl Acad Sci USA 1997; 94: 6369–6374.
Gotoh A, Ko SC, Shirakawa T, Cheon J, Kao C, Miyamoto T et al. Development of prostate-specific antigen promoter-based gene therapy for androgen-independent human prostate cancer. J Urol 1998; 60: 220–229.
Yu D, Jia WW, Gleave ME, Nelson CC, Rennie PS . Prostate-tumour targeting of gene expression by lentiviral vectors containing elements of the probasin promoter. Prostate 2004; 59: 370–382.
Yu D, Scott C, Jia WW, Benedetti AD, Williams BJ, Fazli L et al. Targeting and killing of prostate cancer cells using lentiviral constructs containing a sequence recognized by translation factor eIF4E and a prostate-specific promoter. Cancer Gene Ther 2006; 13: 32–43.
Yang CT, Song J, Bu X, Cong YS, Bacchetti S, Rennie PS et al. Herpes simplex virus type-1 infection upregulates cellular promoters and telomerase activity in both tumour and nontumour human cells. Gen Ther 2003; 10: 1494–1502.
Krisky DM, Wolfe D, Goins WF, Marconi PC, Ramakrishnan R, Mata M et al. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gen Ther 1998; 5: 1593–1603.
Hasenson M, Hartley-Asp B, Kihlfors C, Lundin A, Gustafsson JA, Pousette A . Effect of hormones on growth and ATP content of a human prostatic carcinoma cell line, LNCaP-r. Prostate 1985; 7: 183–194.
Curi MA, Skelly CL, Meyerson SL, Baldwin ZK, Balasubramanian V, Advani SJ et al. Sustained inhibition of experimental neointimal hyperplasia with a genetically modified herpes simplex virus. J Vasc Surg 2003; 37: 1294–1300.
Kochl S, Niederstatter H, Parson W . DNA extraction and quantitation of forensic samples using the phenol–chloroform method and real-time PCR. Methods Mol Biol 2005; 297: 13–30.
Park F, Ohashi K, Kay MA . The effect of age on hepatic gene transfer with self-inactivating lentiviral vectors in vivo. Mol Ther 2003; 8: 314–323.
Spaete RR, Frenkel N . The herpes virus amplicon: a new eukaryotic defective-virus cloning-amplifying vector. Cell 1982; 30: 295–304.
Geller AI, Breakefield XO . A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons. Science 1988; 241: 1667–1669.
DeLuca NA, McCarthy AM, Schaffer PA . Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediately-early regulatory protein ICP4. J Virol 1985; 56: 558–570.
Hummel JL, Safroneeva E, Mossman KL . The role of ICP0-Null HSV-1 and interferon signaling defects in the effective treatment of breast adenocarcinoma. Mol Ther 2005; 12: 1101–1110.
Glauser DL, Ackermann M, Saydam O, Fraefel C . Chimeric herpes simplex virus/adeno-associated virus amplicon vectors. Curr Gene Ther 2006; 6: 315–324.
Acknowledgements
We thank Ms Yang Hang (Brain Research Centre, University of British Columbia Hospital) and Mrs Mary Bowden (Prostate Centre at Vancouver General Hospital) for their technical assistance with the animal work and Mr Robert Bell, Head of Bioinformatics at the Prostate Centre, for help with statistical analyses. This research was supported by grants from the Terry Fox Foundation/National Cancer Institute of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CF., Bu, L., Rennie, P. et al. An HSV-1 amplicon system for prostate-specific expression of ICP4 to complement oncolytic viral replication for in vitro and in vivo treatment of prostate cancer cells. Cancer Gene Ther 14, 652–660 (2007). https://doi.org/10.1038/sj.cgt.7701052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701052
Keywords
This article is cited by
-
Oncolytic virotherapy for urological cancers
Nature Reviews Urology (2016)
-
Intravesical treatment of advanced urothelial bladder cancers with oncolytic HSV-1 co-regulated by differentially expressed microRNAs
Gene Therapy (2016)
-
Transcriptional and Translational Dual-regulated Oncolytic Herpes Simplex Virus Type 1 for Targeting Prostate Tumors
Molecular Therapy (2010)
-
Lentiviruses with trastuzumab bound to their envelopes can target and kill prostate cancer cells
Cancer Gene Therapy (2009)