Abstract
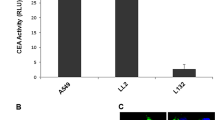
Squamous cell carcinoma antigens SCCA1 and SCCA2 are highly homologous serine proteinase inhibitors which have been widely utilized as serological markers for squamous cell cancers, but it has recently been demonstrated that only SCCA2 is truly specific for certain forms of lung cancer. Using a construct containing the 5′-flanking region of the SCCA2 gene between −460 and +0 bp and the luciferase reporter gene, SCCA2 promoter activity was detected in SCCA2-producing SCC cell lines (LK-2, LC-1), but not in SCCA2-nonproducing lung adenocarcinoma cell lines (A549, ABC-1, and RERF-LC-MS) or normal cells (WI-38, SAEC, and NHEK-Adult). Infection with a recombinant adenovirus vector, Ad-SCCA2-DsRed, resulted in cell-specific expression of the SCCA2 promoter-driven DsRed marker gene only in LK-2 and LC-1 cells. The same strategy was used for SCCA2-driven expression of a proapoptotic gene, (KLAKLAK)2, which can cause mitochondrial disruption by triggering mitochondrial permeabilization and swelling, resulting in the release of cytochrome c and induction of apoptosis. Infection with Ad-SCCA2-KLAKLAK2 specifically reduced the growth of the two human lung SCC cell lines compared to the SCCA2 nonproducing cell lines both in vitro and in vivo, suggesting that the SCCA2 promoter had a tumor-specific effect. These results suggest that transduction of SCCA2 promoter-controlled suicide genes by adenoviral vectors can confer transcriptionally targeted cytotoxicity in SCCA2-producing lung SCC cells, and represents a novel strategy for gene transfer specifically targeted to SCC in the lung.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Levi F, Lucchini F, Negri E, La Vecchia C . Worldwide patterns of cancer mortality, 1990–1994. Eur J Cancer Prev 1999; 8: 381–400.
Ginseberg R, Vokes E, Raben A . Cancer of the lung: non-small-cell lung cancer. In: De Vita V, Hellman S, Rosenberg S (eds). Cancer: Principles and Practice of Oncology, 5th edn. Lippincott: Philadelphia, 1997, pp 858–911.
Greenlee RT, Hill-Harmon MB, Murray T, Thun M . Cancer statistics. CA Cancer J Clin 2001; 51: 15–36.
Osaki T, Tanio Y, Tachibana I, Hosoe S, Kumagai T, Kawase I et al. Gene therapy for carcinoembryonic antigen-producing human lung cancer cells by cell type-specific expression of herpes simplex virus thymidine kinase gene. Cancer Res 1994; 54: 5258–5261.
Takeuchi M, Shichinohe T, Senmaru N, Miyamoto M, Fujita H, Takimoto M et al. The dominant-negative H-ras mutant, N116Y, suppresses growth of metastatic human pancreatic cancer cells in the liver of nude mice. Gene Ther 2000; 7: 518–526.
Kato H, Torigoe T . Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977; 40: 1621–1628.
Takeshima N, Suminami Y, Takeda O, Abe H, Okuno N, Kato H . Expression of mRNA of SCC antigen in squamous cells. Tumour Biol 1992; 13: 338–342.
Sanchez De Cos J, Masa F, de la Cruz JL, Disdier C, Vergara C . Squamous cell carcinoma antigen (SCC Ag) in the diagnosis and prognosis of lung cancer. Chest 1994; 105: 773–776.
Cheah PL, Liam CK, Yap SF, Looi LM . Squamous cell carcinoma antigen as an adjunct tumour marker in primary carcinoma of the lung. J Clin Pathol 1994; 47: 535–537.
Mino N, Iio A, Hamamoto K . Availability of tumor-antigen 4 as a marker of squamous cell carcinoma of the lung and other organs. Cancer 1988; 62: 730–734.
Bolli JA, Doering DL, Bosscher JR, Day Jr TG, Rao CV, Owens K et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol 1994; 55: 169–173.
Kato H, Nagaya T, Torigoe T . Heterogeneity of a tumor antigen TA-4 of squamous cell carcinoma in relation to its appearance in the circulation. Gann 1984; 75: 433–435.
Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD et al. A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA 1995; 92: 3147–3151.
Murakami A, Suminami Y, Sakaguchi Y, Nawata S, Numa F, Kishi F et al. Specific detection and quantitation of SCC antigen 1 and SCC antigen 2 mRNAs by fluorescence-based asymmetric semi-nested reverse transcription PCR. Tumour Biol 2000; 21: 224–234.
Abe H, Okuno N, Takeda O, Suminami Y, Kato H, Nakamura K . Analysis on heterogeneity of squamous cell carcinoma antigen by two-dimensional electrophoresis. Electrophoresis 1994; 15: 988–991.
Stenman J, Hedstrom J, Grenman R, Leivo I, Finne P, Palotie A et al. Relative levels of SCCA2 and SCCA1 mRNA in primary tumors predicts recurrent disease in squamous cell cancer of the head and neck. Int J Cancer 2001; 95: 39–43.
Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA et al. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem 1997; 272: 1849–1855.
Sakaguchi Y, Kishi F, Murakami A, Suminami Y, Kato H . Structural analysis of human SCC antigen 2 promoter. Biochim Biophys Acta 1999; 1444: 111–116.
Hamada K, Shinomiya H, Asano Y, Kihana T, Iwamoto M, Hanakawa Y et al. Molecular cloning of human squamous cell carcinoma antigen 1 gene and characterization of its promoter. Biochim Biophys Acta 2001; 1518: 124–131.
Javadpour MM, Juban MM, Lo WC, Bishop SM, Alberty JB, Cowell SM et al. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem 1996; 39: 3107–3113.
Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 1999; 5: 1032–1038.
Mai JC, Mi Z, Kim SH, Ng B, Robbins PD . A proapoptotic peptide for the treatment of solid tumors. Cancer Res 2001; 61: 7709–7712.
Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 1998; 72: 4756–4764.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Hitt M, Bett AJ, Addison C, Prevec L, Graham FL . Techniques for human adenovirus vector construction and characterization. In: Adolph K (ed). Methods in Molecular Genetics. Elsevier Science & Technology Books: San Diego, Academic Press, 1995, pp 13–30.
Carlsson G, Gullberg B, Hafstrom L . Estimation of liver tumor volume using different formulas – an experimental study in rats. J Cancer Res Clin Oncol 1983; 105: 20–23.
Kagawa S, Pearson SA, Ji L, Xu K, McDonnell TJ, Swisher SG et al. A binary adenoviral vector system for expressing high levels of the proapoptotic gene bax. Gene Ther 2000; 7: 75–79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshikiri, T., Miyamoto, M., Hiraoka, K. et al. Transcriptional targeting of adenovirus vectors with the squamous cell carcinoma-specific antigen-2 promoter for selective apoptosis induction in lung cancer. Cancer Gene Ther 13, 856–863 (2006). https://doi.org/10.1038/sj.cgt.7700953
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700953