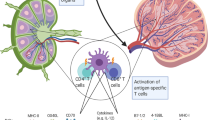
Abstract
Most cancer vaccines to date have made use of common tumor antigens or allogenic cancer cell lines. The majority of tumor antigens may, however, be unique patient-specific antigens. Dendritic cells (DCs) are the most potent antigen-presenting cells known. The present report is a full-scale preclinical evaluation of autologous DCs transfected with autologous tumor-mRNA (tDCs) for vaccination in malignant melanoma. By using autologous tumor-mRNA, we intend to make the DCs present a broad spectrum of tumor-associated antigens relevant to each individual patient. Previously, we have described effective methods for mRNA-transfection into DCs by square-wave electroporation and for generating large numbers of DCs. Here, we demonstrate the ability of tDCs, made under full-scale vaccine conditions, to generate in vitro T-cell responses specific for antigens encoded by the transfected tumor-mRNA. T-cell proliferation assays demonstrated tDC-specific responses for all six patients tested. Responses were further studied by IFNγ ELISPOT and Bioplex cytokine assays (two patients) and by experiments on isolated CD4+ and CD8+ T cells, including HLA-blockage (one patient). Moreover, we describe the results of extensive tumor-RNA analysis using Agilent Bioanalyser, a method that we have implemented in the clinical protocol. Based on this preclinical evaluation, a vaccine trial has been started.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Eigentler TK, Caroli UM, Radny P, Garbe C . Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759.
Parmiani G, Anichini A, Fossati G . Cellular immune response against autologous human malignant melanoma: are in vitro studies providing a framework for a more effective immunotherapy? J Nat Cancer Inst. 1990;82:361–370.
Clemente CG, Mihm Jr MC, Bufalino R, Zurrida S, Collini P, Cascinelli N . Prognostic value of tumour infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310.
Marchand M, Weynants P, Rankin E, et al. Tumour regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883–885.
Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327.
Wang F, Bade E, Kuniyoshi C, et al. Phase I trial of a MART-1 peptide vaccine with incomplete Freund's adjuvant for resected high-risk melanoma. Clin Cancer Res. 1999;5:2756–2765.
Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E . Induction of tumour immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumour cells. Cancer Res. 2000;60:1028–1034.
Srivastava PK . Do human cancers express shared protective antigens? Or the necessity of remembrance of things past. Semin Immunol. 1996;8:295–302.
Slingluff CL . Targeting unique tumor antigens and modulating the cytokine environment may improve immunotherapy for tumors with immune escape mechanisms. Cancer Immunol. immunother. 1999;48:371–373.
Anichini A, Mortarini R, Maccalli C, et al. Cytotoxic T cells directed to tumour antigens not expressed on normal melanocytes dominate HLA-A2.1-restricted immune repertoire to melanoma. J Immunol. 1996;156:208–217.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature. 1998;392:245–252.
Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumour lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332.
Schuler G, Schuler-Thurner B, Steinman RM . The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147.
Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458.
Saebøe-Larssen S, Fossberg E, Gaudernack G . mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J Immunol Methods. 2002;259:191–203.
Mu LJ, Gaudernack G, Saebøe-Larssen S, Hammerstad H, Tierens A, Kvalheim G . A protocol for generation of clinical grade mRNA-transfected monocyte-derived dendritic cells for cancer vaccines. Scandi J Immunol. 2003;58:578–586.
Mueller O, Hahnenberger K, Dittmann M, et al. A microfluidic system for high-speed reproducible DNA sizing and quantitation. Electrophoresis. 2000;21:128–134.
Gottwald E, Müller O, Polten A . Semiquantitative reverse transcription-polymerase chain reaction with the Agilent 2100 Bioanalyzer. Electrophoresis. 2001;22:4016–4022.
Liu CH, Ma WL, Shi R, Zhang B, Ou YQ, Zheng WL . Gene expression study of Saccharomyces cerevisiae with the Agilent 2100 bioanalyser. Br J Biomed Sci. 2003;60:22–25.
Ricicova M, Palkova Z . Comparative analyses of Saccharomyces cervesiae RNAs using Agilent RNA 6000 Nano Assay and agarose gel electrophoresis. FEMS Yeast Res. 2003;4:119–122.
Nussenzweig MC, Steinman RM . Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980;151:1196–1212.
Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M . Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–772.
Smolen JS, Chused TM, Novotny EA, Steinberg AD . The human autologous mixed lymphocyte reaction. III. Immune circuits. J Immunol. 1982;129:1050–1053.
Steinberg AD, Smith HR, Laskin CA, Steinberg BJ, Smolen JS . Studies of immune abnormalities in systemic lupus erythematosus. Am J Kidney Dis. 1982;2:101–110.
Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811.
O’Neill DW, Adams, S, Bhardwaj N . Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246.
Rollins BJ . Chemokines. Blood. 1997;90:909–928.
De Vries IJ, Krooshoop DJ, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17.
Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK . Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58.
Figdor CG, De Vries IJ, Lesterhuis WJ, Melief CJ . Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480.
Acknowledgements
This work was supported by the Norwegian Ministry of Health (Gene Therapy Fund) and the Norwegian Cancer Society. The authors thank Ms Hege Hammerstad, Ms Inger-Lise Haakensen and Mrs Anne Brunsvig for excellent work on DC generation, and the research nurses Kristin Øwre and Lone Hegg for excellent patient care. We also thank Dr Carrie Grimsrud for helpful contribution on total RNA extraction and Professor Olav Kaalhus for valuable advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kyte, J., Kvalheim, G., Aamdal, S. et al. Preclinical full-scale evaluation of dendritic cells transfected with autologous tumor-mRNA for melanoma vaccination. Cancer Gene Ther 12, 579–591 (2005). https://doi.org/10.1038/sj.cgt.7700837
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700837
Keywords
This article is cited by
-
Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer
Cancer Immunology, Immunotherapy (2017)
-
Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination
Cancer Immunology, Immunotherapy (2015)
-
Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma
Cancer Immunology, Immunotherapy (2013)
-
hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes
Cancer Immunology, Immunotherapy (2011)
-
High immunogenic potential of p53 mRNA-transfected dendritic cells in patients with primary breast cancer
Breast Cancer Research and Treatment (2011)