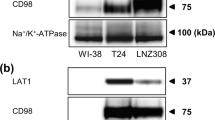
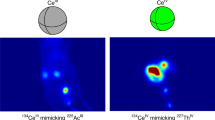
Abstract
In order to noninvasively detect Salmonella delivery vectors within tumors, we used a genetically modified Salmonella, VNP20009, that expresses the herpes simplex thymidine kinase (HSV1-tk) reporter gene. VNP20009-TK were able to selectively localize within murine tumor models and to effectively sequester a radiolabeled nucleoside analogue, 2′-fluoro-1-β-D-arabino-furanosyl-5-iodo-uracil (FIAU). A quantitative relationship between the level of radioactivity accumulated and the number of bacteria in tumor and different tissues was demonstrated. The in vivo accumulation of [14C]FIAU measured in tissue sample homogenates and sections were related to Salmonella number and to immunohistochemical bacterial staining, respectively. Quantitative autoradiography (QAR) revealed the relative intensity of [14C]FIAU accumulation in a tumor cross-section, demonstrating that the peripheral region of the tumor was significantly less active than internal regions. [124I]FIAU positron emission tomography (PET) and subsequent tissue radioactivity and bacterial concentration measurements were compared. A log–log relationship was found, and the PET images could identify multiple tumor sites. The ability to noninvasively detect Salmonella vectors by PET imaging has the potential to be conducted in a clinical setting, and could aid in development of these vectors by demonstrating the efficiency and duration of targeting as well as indicating the locations of tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pawelek J, Low KB, Bermudes D . Bacteria as tumour-targeting vectors. Lancet Oncol Rev. 2003; 4: 548–556.
Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S . Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000; 7: 269–274.
Yazawa K, Fujimori M, Nakamura T, et al. Bifidobacterium longum as a delivery system for cancer gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat. 2001; 66: 165–170.
Li X, Fu G-F, Fan Y-R, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003; 10: 105–111.
Minton NP, Mauchline ML, Lemmon MJ, et al. Chemotherapeutic tumour targeting using clostridial spores. FEMS Microbiol Rev. 1995; 17: 357–364.
Fox ME, Lemmon MJ, Mauchline ML, et al. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Therapy. 1996; 3: 173–178.
Lemmon MJ, van Zijl P, Fox ME, et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Therapy. 1997; 8: 791–796.
Theys J, Landuyt W, Nuyts S, et al. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther. 2001; 8: 294–297.
Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B . Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA. 2001; 98: 15155–15160.
Nuyts S, Mellaert IV, Theys J, Landuyt W, Lambin P, Anne J . Clostridium spores for tumor-specific drug delivery. Anti-Cancer Drugs. 2002; 13: 115–125.
Nuyts S, Van Mellaert L, Theys J, et al. Radio-responsive recA promoter significantly increases TNFα production in recombinant clostridia after 2 Gy irradiation. Gene Therapy. 2002; 8: 1197–1201.
Liu S-C, Minton NP, Giaccia AJ, Brown JM . Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Therapy. 2002; 9: 291–296.
Pawelek JM, Low KB, Bermudes D . Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997; 57: 4537–4544.
Low KB, Ittensohn M, Le T, et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol. 1999; 17: 37–41.
Platt J, Sodi S, Kelley M, et al. Antitumour effects of genetically engineered Salmonella in combination with radiation. Eur J Cancer. 2000; 36: 2397–2402.
Luo X, Li Z, Lin S, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res. 2001; 12: 501–508.
Rosenberg SA, Spiess PM, Kleiner DE . Antitumour effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother. 2002; 25: 218–225.
Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004; 22: 313–320.
Pawelek JM, Sodi S, Chakraborty AK, et al. Salmonella pathogenicity Island-2 and anticancer activity in mice. Cancer Gene Ther. 2002; 9: 813–818.
Chen LM, Kaniga K, Galán JE . Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 1996; 21: 1101–1115.
Monack DM, Raupach B, Hromockyj AE, Falkow S . Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996; 93: 9833–9838.
Zhou X, Mantis N, Zhang XR, Potoka DA, Watkins SC, Ford HR . Salmonella typhimurium induces apoptosis in human monocyte-derived macrophages. Microbiol Immunol. 2000; 44: 987–995.
Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000; 181: 1996–2002.
Lee KC, Zheng L-M, Xuo X, et al. Comparative evaluation of the acute toxic effects in monkeys, pigs, and mice of a genetically engineered Salmonella strain (VNP20009) being developed as an antitumor agent. Int J Toxicol. 2000; 19: 19–25.
Zheng L-M, Luo X, Feng M, et al. Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncol Res. 2000; 12: 127–135.
Bermudes D, Low KB, Pawelek J, et al. Tumour-selective Salmonella-based cancer therapy. Biotechnol Genet Eng Rev. 2001; 18: 219–233.
Toso J, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002; 20: 142–152.
Nemunaitis J, Cunningham C, Senzer N, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003; 10: 737–744.
Dresselaers T, Theys J, Nuyts S, et al. Non-invasive 19F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br J Cancer. 2003; 89: 1796–1801.
Tjuvajev J, Blasberg R, Luo X, Zheng L-M, King I, Bermudes D . Salmonella-based tumor-targeted cancer therapy: tumor amplified protein expression therapy (TAPET™) for diagnostic imaging. Control Release Soc. 2001; 74: 313–315.
Qiao J, Doubrovin M, Sauter MV, et al. Tumor-specific transcriptional targeting of suicide gene therapy. Genet Ther. 2002; 9: 168–175.
Tjuvajev JG, Chen SH, Joshi A, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999; 59: 5186–5193.
Bennett JJ, Tjuvajev JG, Johnson P, et al. Positron emission tomography imaging for herpes virus infection: implications for oncolytic viral treatments of cancer. Nat Med. 2001; 7: 859–863.
Jacobs A, Tjuvajev JG, Doubrovin M, et al. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001; 61: 2983–2995.
Tjuvajev JG, Stockhammer G, Desai R, et al. Imaging the expression of transfected genes in vivo. Cancer Res. 1995; 55: 6126–6132.
Tjuvajev JG, Finn R, Watanabe K, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996; 56: 4087–4095.
Tjuvajev JG, Avril N, Oku T, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998; 58: 4333–4341.
Tjuvajev JG, Doubrovin M, Akhurst T, et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 2002; 43: 1072–1083.
Hackman T, Doubrovin M, Balatoni J, et al. Imaging expression of cytosine deaminase – herpes virus thymidine kinase fusion gene (CD/TK) expression with [124I]FIAU and PET. Mol Imag. 2002; 1: 36–42.
Low KB, Ittensohn M, Luo X, et al. Construction of VNP20009, a novel, genetically stable antibiotic sensitive strain of tumor-targeting Salmonella for parentral administration in humans. In: Springer C, ed. Suicide Gene Therapy: Methods and Protocols. Totowa, NJ: Humana Press; 2003: 47–59.
Murray SR, Bermudes D, de Felipe SW, Low KB . Extragenic suppressors of msbB- growth defects in Salmonella. J Bacteriol. 2001; 183: 5554–5561.
Bermudes D, Low B, Pawelek J . Tumor-targeted Salmonella: strain development and expression of the HSV TK effector gene. In: Walther W, and Stein U, eds. Gene Therapy: Methods and Protocols, Vol 35. Totowa, NJ: Humana Press; 419–436.
Summers WC, Summers WP . [125I]deoxycytidine used in a rapid, sensitive, and specific assay for herpes simplex virus type 1 thymidine kinase. J Virol. 1977; 24: 314–318.
Sheh Y, Koziorowski J, Balatoni J, et al. Low energy cyclotron production and chemical separation of “no carrier added” iodine-124 from a reusable, enriched tellurium-124 dioxide/aluminum oxide solid solution target. Radiochim Acta. 2000; 88: 169–173.
Tjuvajev JG, Doubrovin M, Akhurst T, et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 2002; 43: 1072–1083.
Blasberg RG, Groothuis D, Molnar P . Application of quantitative autoradiographic measurements in experimental brain tumor models. Semin Neurol. 1981; 1: 203–223.
Acknowledgements
We thank Terry Doyle, Ivan King, and Mario Sznol for helpful discussions. This work was supported by National Cancer Institute contract N01-CO-07102, NIH Grants P50 CA86438 and R24 CA83084, and Vion Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soghomonyan, S., Doubrovin, M., Pike, J. et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene Ther 12, 101–108 (2005). https://doi.org/10.1038/sj.cgt.7700779
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700779
Keywords
This article is cited by
-
Engineered live bacteria as disease detection and diagnosis tools
Journal of Biological Engineering (2023)
-
Recent Progress in the Molecular Imaging of Tumor-Treating Bacteria
Nuclear Medicine and Molecular Imaging (2021)
-
Salmonella Typhimurium as an Anticancer Therapy: Recent Advances and Perspectives
Current Clinical Microbiology Reports (2019)
-
Salmonella-Mediated Cancer Therapy: Roles and Potential
Nuclear Medicine and Molecular Imaging (2017)
-
Violacein as a genetically-controlled, enzymatically amplified and photobleaching-resistant chromophore for optoacoustic bacterial imaging
Scientific Reports (2015)