Abstract
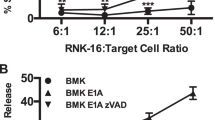
Inducing Fas-mediated apoptosis in prostate cancer (PCa) is a promising new therapeutic approach with the potential to overcome delivery issues currently problematic in cancer gene therapy. We have previously demonstrated that a Fas Ligand (FasL) expressing adenovirus (AdGFPFasLTET) was able to induce Fas-mediated apoptosis in a panel of PCa cell lines regardless of their Fas-sensitivity as determined by the agonistic Fas antibody CH-11. We now report that AdGFPFasLTET-infected cells produce apoptotic bodies and cellular debris that continues to elicit FasL-mediated bystander killing in uninfected neighboring cells. Using light microscopy, we demonstrate that AdGFPFasLTET-infected cells release apoptotic bodies and cellular debris into the local environment and that this material will induce bystander killing in Jurkat, PPC-1, and PC-3 target cells, but not in DU145 and K-562 cells. The bystander killing mechanism is mediated through Fas/FasL interaction because it is significantly inhibited if target cells are pretreated with the pan spectrum caspase inhibitor Z-VAD-FMK or the Fas neutralizing antibody ZB-4. Coincubation of PPC-1 target cells with apoptotic bodies and cellular debris (effector material) induce nearly complete target cell killing at a ratio of 1:1 target to effector. Collectively, these data indicate that AdGFPFasLTET-infected PCa cells release apoptotic and cellular debris capable of inducing bystander killing in PCa and supports the development of FasL as a gene therapy agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Heise C, Ganly I, Kim YT, et al. Efficacy of a replication-selective adenovirus against ovarian carcinomatosis is dependent on tumor burden, viral replication and p53 status. Gene Ther. 2000;7:1925–1926.
Haviv YS, Curiel DT . Conditional gene targeting for cancer gene therapy. Adv Drug Deliv Rev. 2001;53:135–154.
Galanis E, Vile R, Russell SJ . Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol. 2001;38:177–192.
Suda T, Takahashi T, Golstein P, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178.
Yonehara S, Ishii A, Yonehara M . A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756.
Trauth BC, Klas C, Peters AM, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305.
Hyer ML, Voelkel-Johnson C, Rubinchik S, et al. Intracellular fas ligand expression causes fas-mediated apoptosis in human prostate cancer cells resistant to monoclonal antibody-induced apoptosis. Mol Ther. 2000;2:348–358.
Hyer ML, Sudarshan S, Kim Y, et al. Down-regulation of c-FLIP sensitizes DU145 prostate cancer cells to Fas-mediated apoptosis. Cancer Biol Ther. 2002;1:405–410.
Rubinchik S, Ding R, Qiu A J, et al. Adenoviral vector which delivers FasL-GFP fusion protein regulated by the tet-inducible expression system. Gene Ther. 2000;7:875–885.
Schneider P, Holler N, Bodmer JL, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213.
Douglas JT, Rogers BE, Rosenfeld ME, et al. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578.
Hedlund TE, Meech SJ, Srikanth S, et al. Adenovirus-mediated expression of Fas ligand induces apoptosis of human prostate cancer cells. Cell Death Differ. 1999;6:175–182.
Jodo S, Xiao S, Hohlbaum A, et al. Apoptosis-inducing membrane vesicles. A novel agent with unique properties. J Biol Chem. 2001;276:39938–39944.
Beltinger C, Fulda S, Kammertoens T, et al. Herpes simplex virus thymidine kinase/gancyclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci USA. 1999;96:8699–8704.
Wei SJ, Chao Y, Shih YL, et al. Involvement of Fas (CD95/APO-1) and Fas ligand in apoptosis induced by gancyclovir treatment of tumor cells transduced with herpes simplex virus thymidine kinase. Gene Ther. 1999;6:420–431.
Hall SJ, Canfield SE, Yan Y, et al. A novel bystander effect involving tumor cell-derived Fas and FasL interactions following Ad.HSV-tk and Ad.mIL-12 gene therapies in experimental prostate cancer. Gene Ther. 2002;9:511–517.
Fukazawa T, Fujiwara T, Morimoto Y, et al. Differential involvement of the CD95 (Fas/APO-1) receptor/ligand system on apoptosis induced by the wild-type p53 gene transfer in human cancer cells. Oncogene. 1999;18:2189–2199.
Eaton JD, Perry MJA, Todryk SM, et al. Genetic prodrug activation therapy (GPAT) in two rat prostate models generates an immune bystander effect and can be monitored by magnetic resonance techniques. Gene Ther. 2001;8:557–567.
Rubinchik S, Wang D, Yu H, et al. A complex adenovirus vector that delivers FasL-GFP with combined prostate-specific and tetracycline-regulated expression. Mol Ther. 2001;4:416–426.
Lowe SL, Rubinchik S, Honda Y, et al. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001;8:1363–1371.
Acknowledgements
Supported by NIH CA 69596,CA 88163 and DOD Grant N6311601MD10004. We thank Rick Peppler and the MUSC flow cytometry facility for acquiring the flow cytometry data. We especially thank Joanne Douglas for kindly providing the adenovirus neutralizing antibody 1D6.14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyer, M., Sudarshan, S., Schwartz, D. et al. Quantification and characterization of the bystander effect in prostate cancer cells following adenovirus-mediated FasL expression. Cancer Gene Ther 10, 330–339 (2003). https://doi.org/10.1038/sj.cgt.7700576
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700576
Keywords
This article is cited by
-
Enhancing the bystander killing effect of an oncolytic HSV by arming it with a secretable apoptosis activator
Gene Therapy (2015)
-
International Society for Cell and Gene Therapy of Cancer: 2005 meeting in Shenzhen, China
Cancer Gene Therapy (2007)
-
The effects of FasL on inflammation and tumor survival are dependent on its expression levels
Cancer Gene Therapy (2007)
-
FasL gene therapy: a new therapeutic modality for head and neck cancer
Cancer Gene Therapy (2006)
-
Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report
Cancer Gene Therapy (2006)