Abstract
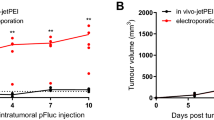
Electroporation-mediated gene transfer relies upon direct delivery of plasmids into cells permeabilized by electric fields, a method more efficient than transfer using nonviral vectors, although neither approaches the transfer efficiency of viral vectors. Here we studied electrotransfer of a gene encoding an angiogenesis inhibitor (endostatin) into primary tumors and muscle tissues, which would serve as a site of synthesis and secretion into the bloodstream of a therapeutic antimetastatic protein with systemic effects. Optimum electroporation conditions (voltage, number and duration of impulses, separation of caliper electrodes) were first established to maximize expression of a reporter gene transferred into murine Renca kidney carcinoma, B16(F10) melanoma, or skeletal muscle tissues. In neoplastic tissues, electrotransfer of plasmid DNA was far more efficient than electroporation with lipoplexes, but no differences between naked DNA and lipoplexes were found in case of electroporated muscles. We then studied the electrotransfer of plasmid DNA carrying the endostatin gene into pre-established experimental Renca tumors. A significant inhibition of tumor growth was observed in animals electroporated with this construct. Electrotransfer of the endostatin gene into muscle tissues resulted in reduced numbers of experimental B16(F10) metastases in the lungs. This study clearly shows that electroporation may be used to efficiently transfer antiangiogenic genes into both normal and neoplastic tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Somia N, Verma IM . Gene therapy: trials and tribulations Nat Rev 2000 1: 91–99
Mountain A . Gene therapy: the first decade Trends Biotechnol 2000 18: 119–128
Kay MA, Glorioso JC, Naldini L . Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics Nat Med 2001 7: 33–40
Nishikawa M, Huang L . Nonviral vectors in the new millennium: delivery barriers in gene transfer Hum Gene Ther 2001 12: 861–870
Gómez-Navarro J, Curiel DT, Douglas JT . Gene therapy for cancer Eur J Cancer 1999 35: 2039–2057
Vile RG, Russell SJ, Lemoine NR . Cancer gene therapy: hard lessons and new courses Gene Ther 2000 7: 2–8
Folkman J . Antiangiogenic gene therapy Proc Natl Acad Sci USA 1998 95: 9064–9066
Kong H-L, Crystal RG . Gene therapy for tumor angiogenesis J Natl Cancer Inst 1998 90: 273–286
Gasparini G . The rationale and future potential of angiogenesis inhibitors in neoplasia Drugs 1999 58: 17–38
Feldman AL, Libutti SK . Progress in antiangiogenic gene therapy of cancer Cancer 2000 89: 1181–1194
Folkman J . New perspectives in clinical oncology from angiogenesis research Eur J Cancer 1996 32A: 2534–2539
Carmeliet P, Jain RK . Angiogenesis in cancer and other diseases Nature 2000 407: 249–257
O'Reilly M, Boehm T, Shing Y et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth Cell 1997 88: 277–285
Dhanabal M, Ramchandran R, Waterman MJF et al. Endostatin induces endothelial cell apoptosis J Biol Chem 1999 274: 11721–11726
Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF . Adeno-associated virus–mediated delivery of antiangiogenic factors as an antitumor strategy Cancer Res 1998 58: 5673–5677
Feldman AL, Restifo NP, Alexander HR et al. Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice Cancer Res 2000 60: 1503–1506
Blezinger P, Wang J, Gondo M et al. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene Nat Biotechnol 1999 17: 343–348
Chen Q-R, Kumar D, Stass SA, Mixson AJ . Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice Cancer Res 1999 59: 3308–3312
Szary J, Szala S . Intra-tumoral administration of naked plasmid DNA encoding mouse endostatin inhibits renal carcinoma growth Int J Cancer 2001 91: 835–839
Mir LM . Therapeutic perspectives of in vivo cell electropermeabilization Bioelectrochemistry 2000 53: 1–10
Gehl J, Sørensen TH, Nielsen K et al. In vivo electroporation of skeletal muscle: threshold, efficacy and relation to electric field distribution Biochim Biophys Acta 1999 1428: 233–240
Bureau MF, Gehl J, Deleuze V, Mir LM, Scherman D . Importance of association between permeabilization and electrophoretic forces intramuscular DNA electrotransfer Biochim Biophys Acta 2000 1474: 353–359
Hartikka J, Sawdey M, Cornefert-Jensen F et al. An improved plasmid DNA expression vector for direct injection into skeletal muscle Hum Gene Ther 1996 7: 1205–1217
Horn NA, Meek JA, Budahazi G, Marquet M . Cancer gene therapy using plasmid DNA: purification of DNA for human clinical trials Hum Gene Ther 1995 6: 565–573
Gao X, Huang L . A novel cationic liposome reagent for efficient transfection of mammalian cells Biochem Biophys Res Commun 1991 179: 280–285
Szala S, Missol E, Sochanik A, Strozyk M . The use of cationic liposomes DC-Chol/DOPE and DDAB/DOPE for direct transfer of Escherichia coli cytosine deaminase gene into growing melanoma tumors Gene Ther 1996 3: 1026–1031
Hogan B, Constantini F, Lacy E . Manipulating the Mouse Embryo A Laboratory Manual Cold Spring Harbor Laboratory, New York 1986
Im S-Y, Ko H-M, Han S-J et al. Prevention of tumor growth and metastasis by interleukin (IL)-10 transfection: a potent inhibitory role of IL-10 on angiogenesis Mol Cell 1996 6: 473–480
Budryk M, Cichoń T, Szala S . Direct in vivo transfer of plasmid DNA into murine tumors: effects of endotoxin presence and transgene localization Acta Biochim Pol 2001 48: 795–800
Clark PR, Stopeck AT, Ferrari M, Parker SE, Hersh EM . Studies of direct intratumoral gene transfer using cationic lipid–complexed plasmid DNA Cancer Gene Ther 2000 7: 853–860
Lohr F, Lo DY, Zaharoff DA et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation Cancer Res 2001 61: 3281–3284
Vicat JM, Boisseau S, Jourdes P et al. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression Hum Gene Ther 2000 11: 909–916
Wells JM, Li LH, Sen A, Jahreis GP, Hui SW . Electroporation-enhanced gene delivery in mammary tumors Gene Ther 2000 7: 541–547
Hanna E, Zhang X, Woodlis J, Breau R, Suen J, Li S . Intramuscular electroporation delivery of IL-12 gene for treatment of squamous cell carcinoma located at distant site Cancer Gene Ther 2001 8: 151–157
Rizzuto G, Cappelletti M, Mennuni C et al. Gene electrotransfer results in a high-level transduction of rat skeletal muscle and corrects anemia of renal failure Hum Gene Ther 2000 11: 1891–1900
Mir LM, Bureau MF, Gehl J et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses Proc Natl Acad Sci USA 1999 96: 4262–4267
Feldman AL, Libutti SK . Correspondence re: Q-R Chen et al, Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice Cancer Res 1999 59: 3308–3312 Letter; Cancer Res 2000;60:1463
Mixson AJ . Correspondence re: Q-R Chen et al, Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice Cancer Res 1999 59: 3308–3312 Reply; Cancer Res 2000;60:1463–1464
Sauter BV, Martinet O, Zhang W-J, Mandeli J, Woo SLC . Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases Proc Natl Acad Sci USA 2000 97: 4802–4807
Feldman AL, Restifo NP, Alexander HR et al. Antiangiogenic gene therapy of cancer utilizing a recombinant adenovirus to elevate systemic endostatin levels in mice Cancer Res 2000 60: 1503–1506
Chen CT, Lin J, Li Q et al. Antiangiogenic gene therapy for cancer via systemic administration of adenoviral vectors expressing secretable endostatin Hum Gene Ther 2000 11: 1983–1996
Kuo CJ, Farnebo F, Yu EY et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer Proc Natl Acad Sci USA 2001 98: 4605–4610
Jin X, Bookstein R, Wills K et al. Evaluation of endostatin antiangiogenesis gene therapy in vitro and in vivo Cancer Gene Ther 2001 8: 982–989
McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ . Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase — increased expression with reduced muscle damage Gene Ther 2001 8: 1264–1270
Acknowledgements
The authors are grateful to H Paterak and M Krawczyk for their excellent technical assistance. They also thank R Hancock and A Sochanik for helpful discussion and critical reading of the manuscript. This study was supported by Grants: 4P05A 046 15 and Z-KBN004/PO4/98 from the Polish State Committee for Scientific Research (KBN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cichoń, T., Jamroży, L., Glogowska, J. et al. Electrotransfer of gene encoding endostatin into normal and neoplastic mouse tissues: Inhibition of primary tumor growth and metastatic spread. Cancer Gene Ther 9, 771–777 (2002). https://doi.org/10.1038/sj.cgt.7700497
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700497
Keywords
This article is cited by
-
Electrotransfer of single-stranded or double-stranded DNA induces complete regression of palpable B16.F10 mouse melanomas
Cancer Gene Therapy (2013)
-
Plasmid injection and application of electric pulses alter endogenous mRNA and protein expression in B16.F10 mouse melanomas
Cancer Gene Therapy (2010)
-
Canstatin gene electrotransfer combined with radiotherapy: preclinical trials for cancer treatment
Gene Therapy (2008)
-
The effect of the histological properties of tumors on transfection efficiency of electrically assisted gene delivery to solid tumors in mice
Gene Therapy (2007)
-
Electrogene therapy using endostatin, with or without suicide gene therapy, suppresses murine mammary tumor growth and metastasis
Cancer Gene Therapy (2007)