Abstract
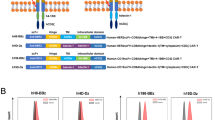
T cells require two distinct signals for optimal activation, an antigen-specific signal, provided by engagement of the T-cell receptor (TCR) and a second costimulatory signal mediated by engagement of CD28 with members of the B7 family. Although infiltrating T cells are present in many malignancies, they appear to be mostly anergic and do not attack the tumor, presumably because of the absence of activation and/or costimulatory signals. Here we describe a novel strategy for the in situ activation of tumor-specific T cells. We genetically modified T cells to secrete a bifunctional fusion protein, comprising the extracellular portion of B7-1 fused to an anticarcinoembryonic antigen (CEA) diabody. In coculture with CEA+ tumor cells autocrine and paracrine secretion of B7-αCEA provided a potent tumor-specific costimulatory signal to T cells in combination with a recombinant αCEA×αCD3 bispecific diabody. B7-αCEA was also found to strongly enhance survival and tumor-specific activation of T cells expressing an anti-CEA TCRζ–based chimeric immune receptor (CIR) both when expressed in cis by the T cells themselves as well as in trans, when added to the culture medium. In the absence of costimulatory signals provided by the tumor, our strategy allows T cells to “arm themselves” by the production of tumor-specific costimulatory proteins. Sustained in situ production of such molecules, like the B7-diabody fusion protein may create a favorable local environment for the activation and proliferation of tumor-reactive T cells and increase the tumoricidal activity of immunotherapeutic approaches targeting the TCR pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Townsend A, Bodmer H . Antigen recognition by class I–restricted T lymphocytes Annu Rev Immunol 1989 7: 601–624
Jenkins MK . The ups and downs of T cell costimulation Immunity 1994 1: 443–446
Tan P, Anasetti C, Hansen JA, Melrose J, Brunraud M, Bradshow J, Ledbetter J, Linsley PS . Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1 J Exp Med 1993 177: 165–173
Mueller DL, Jenkins MK, Schwartz RH . Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy Annu Rev Immunol 1989 7: 445–480
Linsley PS, Ledbetter JA . The role of the CD28 receptor during T cell responses to antigen Annu Rev Immunol 1993 11: 191–212
Allison JP . CD28-B7 interactions in T-cell activation Curr Opin Immunol 1994 6: 414–419
Chen L . Manipulation of T cell responses to tumors by targeting on costimulatory pathway Leukemia 1997 3: 567
Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS . Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4 Cell 1992 71: 1093–1102
Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom KE . Tumor immunogenicity determines the effect of B7 costimulation on T cell–mediated tumor immunity J Exp Med 1994 179: 523–532
Álvarez-Vallina L, Hawkins RE . Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors Eur J Immunol 1996 26: 2304–2309
Álvarez-Vallina L . Targeted cell immunotherapy of human cancer: a promising approach that is extending its initial cell and molecular scope Tumor Targeting 2000 4: 225–234
Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M . Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes J Exp Med 1998 188: 619–626
Eshhar Z, Waks T, Gross G, Schindler DG . Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors Proc Natl Acad Sci USA 1993 90: 720–724
Finney HM, Lawson AD, Bebbington CR, Weir AN . Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product J Immunol 1998 161: 2791–2797
Álvarez-Vallina L . Genetic approaches for antigen-selective therapy Curr Gene Ther 2001 1: 385–397
Gerstmayer B, Altenschmidt U, Hoffmann M, Wels W . Costimulation of T cell proliferation by a chimeric B7-2 antibody fusion protein specifically targeted to cells expressing the erbB2 proto-oncogene J Immunol 1997 158: 4584–4590
Rohrbach F, Gerstmayer B, Biburger M, Wells W . Construction and characterization of bispecific costimulatory molecules containing a minimized CD86 (B7-2) domain and single-chain antibody fragments for tumor targeting Clin Cancer Res 2000 6: 4314–4322
Holliger P, Manzke O, Span M et al. Carcinoembryonic antigen (CEA)-specific T-cell activation in colon carcinoma induced by anti-CD3×anti-CEA bispecific diabodies and B7×anti-CEA bispecific fusion proteins Cancer Res 1999 59: 2909–2916
Marshall KW, Marks JD . Engineering and characterization of a novel fusion protein incorporating B7.2 and an anti–ErbB-2 single-chain antibody fragment for the activation of Jurkat T cells J Immunother 2001 24: 27–36
Rosenberg SA, Spiess P, Lafreniere R . A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes Science 1986 233: 1318–1321
Bolhuis RL, Sturm E, Braakman E . T cell targeting in cancer therapy Cancer Immunol Immunother 1991 34: 1–8
Holliger P, Prospero T, Winter G . “Diabodies”: small bivalent and bispecific antibody fragments Proc Natl Acad Sci USA 1993 90: 6444–6448
Hochuli E, Bannwarth W, Döbeli H, Gentz R, Stüber D . Genetic approach to facilitate purification of recombinant proteins with a novel metal cHeLate adsorbent Biotechnology 1998 6: 1321
Álvarez-Vallina L, Agha-Mohammadi S, Hawkins RE, Rusell SJ . Pharmacological control of antigen responsiveness in genetically modified T lymphocytes J Immunol 1997 159: 5889–5895
Álvarez-Vallina L, González A, Gambón F, Kreisler M, Díaz-Espada F . Delimitation of the proliferative stages in the human thymus indicates that cell expansion occurs before the expression of CD3 (T cell receptor) J Immunol 1993 150: 8–16
Acknowledgements
This work was funded by the Comunidad Autónoma de Madrid Grant 08.1/0016/1998 (to L Álvarez-Vallina). B Blanco is supported by a Comunidad Autónoma de Madrid training Grant 01/0369/2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanco, B., Holliger, P. & Álvarez-Vallina, L. Autocrine costimulation: Tumor-specific CD28-mediated costimulation of T cells by in situ production of a bifunctional B7–anti-CEA diabody fusion protein. Cancer Gene Ther 9, 275–281 (2002). https://doi.org/10.1038/sj.cgt.7700438
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700438
Keywords
This article is cited by
-
Balanced secretion of anti-CEA × anti-CD3 diabody chains using the 2A self-cleaving peptide maximizes diabody assembly and tumor-specific cytotoxicity
Gene Therapy (2017)
-
Inhibition of tumor growth in vivo by in situ secretion of bispecific anti-CEA × anti-CD3 diabodies from lentivirally transduced human lymphocytes
Cancer Gene Therapy (2007)