ABSTRACT
Mot-2 protein is shown to interact with p53 and inhibit its transcriptional activation function. Mot-2 overexpressing stable clones of NIH 3T3 cells were malignantly transformed, however, they had a high level of expression of a p53 downstream gene, p21WAF1. The present study was undertaken to elucidate possible molecular mechanism(s) of such upregulation. An increased level of p21WAF1 expression was detected in stable transfectants although an exogenous reporter gene driven by p21WAF1 promoter exhibited lower activity in these cells suggesting that some post-transcriptional mechanism contributes to upregulation. Western analyses of transient and stable clones revealed that upregulation of p21WAF1 in stable NIH 3T3/mot-2 cells may be mediated by cyclin D1 and cdk-2.
Similar content being viewed by others
INTRODUCTION
Multiple cellular functions of p53, a most frequently mutated tumor suppressor in human cancers, are involved in its ability to induce and regulate cell cycle arrest and apoptosis1,2. Among these are its ability to bind to specific DNA sequences and activate transcription of genes such as, mdm-2, p21WAF1, gadd45, bax and IGFBP-33, 4, 5, 6. p21WAF1, the pioneer member of p21 family of cyclin-cdk inhibitor class of proteins (p21WAF1, p27Kip1 and p57Kip2), that bind to cyclin D17,8 has been implicated as a growth arrest mediator in p53-tumour suppression, cellular senescence and terminal differentiation. Similarly, another class of cyclin-cdk inhibitors, INK4 family members (p16INK4a, p15INK4b, p18INK4c and p19INK4d) that bind to cdk-4 and cdk-69, 10, 11 regulate cell cycle progression. By inactivating the cyclin-cdk complexes, these inhibitors block phosphorylation of RB family of proteins (RB) and abrogate release of E2F (required for cell cycle progression) from RB-E2F complexes12. Number of studies have supported that p21WAF1 functions during normal and induced cellular senescence13, 14, 15, 16, 17. However, mice lacking p21WAF1 have normal phenotype18, 19 and its upregulation has been detected by mitogenic and growth stimulatory signals20,21. These data suggested that physiological function of p21WAF1 may not be limited to execution of cell cycle arrest program.
Mot-2, a member of hsp70 family protein, was seen to interact with p53 and inactivate its transactivation function by abrogating its nuclear translocation 22. While characterizing mouse fibroblasts stably transfected with mot-2, we found that in spite of inactivation of the p53 function by mot-2, these cells have high level of p21WAF1. Studies were therefore undertaken to elucidate the mechanism(s) of such upregulation of p21WAF1. Consistent with the protein levels, Northern analysis revealed an upregulation of p21WAF1. A comparative analysis of p21WAF1, cyclin D1 and cdk-2 in stably- and transiently- transfected cells suggested that the upregulation of p21WAF1 in stable NIH 3T3/mot-2 cells may occur possibly by post-transcriptional and p53-independent mechanism(s) such as those mediated by cyclin D1, cdk-2 and E2F.
MATERIAL AND METHODS
Cell culture
NIH 3T3 cells were transfected (LipofectAMINE™, Life Technologies, Inc) with expression plasmid encoding mouse mot-2 protein and the stable clones were isolated and maintained in 50 μg/ml G418-supplemented growth medium (Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) as described23. Normal mouse embryonic fibroblasts (CMEF) from CD1-ICR mouse and their spontaneously immortalized clone, RS-4, were serially passaged similarly by 1:8 subculturing in DMEM.
Northern blot analysis
RNA from NIH 3T3 and its mot-2 derivatives were extracted by using Isogen (GibcoBRL). Northern analysis for p21WAF1 was performed by using 10 mg of total RNA, separated on 1.5% denaturing agarose gel, transferred to Hybond N (Amersham) membrane by capillary transfer, and probed with the 32P-radiolabeled mouse p21WAF1 cDNA (kindly provided by Dr. Olivia M. Pereira-Smith).
Western blot analysis
The protein sample (10-20 mg) separated on an SDS-poly- acrylamide gel was electroblotted onto a nitrocellulose membrane (BA85, Schleicher and Schuell) using a semidry transfer blotter (Biometra, Tokyo). Immunoassays were performed with anti-mortalin24, -p21 (M-19, Santa Cruz), -p16INK4a (M156, Santa Cruz), -cdk-2 (M2, Santa Cruz), -cdk-4 (C-22, Santa Cruz), -cdk-6 (c-21, Santa Cruz), -cyclin D1 (72-13G, Santa Cruz) or anti-actin (Boehringer Mannheim) antibodies. The immunocomplexes formed were visualized either with horseradish peroxidase (HRP) or alkaline phosphate-conjugated anti-mouse/rabbit immunoglobulin G (IgG) (ECL kit, Amersham Pharmacia Biotech).
RESULTS AND DISCUSSION
NIH 3T3/mot-2 cells show an upregulation of p21WAF1 expression
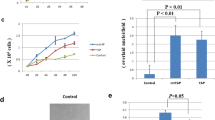
Western analysis with anti-p21 antibody revealed a high expression level of p21WAF1 in NIH 3T3/mot-2 cells as compared to untransfected and vector-transfected cells. This was contrary to the inactivation of p53 function by mot-222,25. We first ascertained that the band detected on Western blot was indeed p21WAF1 by using serially passaged normal mouse embryonic fibroblasts (CMEF) and NIH 3T3 cells cultured at different density (Fig 1A). In agreement to known pattern of p21WAF1 expression in senescence human cells13,26, it was seen to undergo upregulation with in vitro aging of mouse cells. CMEF cells at passage 6-8 showed senescent morphology following which spontaneously immortalized clones emerged. One of such clones, RS-4 that showed mutant p5327 was also assayed for p21 expression level (Fig 1A). In spite of the presence of mutant p53, RS-4 cells showed the p21WAF1 expression, albiet at level less than the one detected in cells at senescent phase (passage 8), suggesting p53-independent expression of p21WAF1. However, p21WAF1 expression in RS-4 cells was not induced by high-density culture in contrast to NIH 3T3 cells that have wild type p53 protein (Fig 1A). These data established that the band detected on our Westerns represented p21WAF1. NIH 3T3/mot-2 cells (two independent clones) showed a high level of expression of p21WAF1 even at low-density culture and did not support inactivation of p53 function by mot-2 in these cells (Fig 1A). A p53-independent increase in the level of p21WAF1 expression of by post-transcriptional28 and post-translational29 mechanism has been reported. Therefore, to detect the level at which p21WAF1 had undergone upregulation in NIH 3T3/mot-2 cells, we next performed Northern analysis. Consistent with Western blot analysis, a high level of transcript was apparent in NIH 3T3/mot-2 cells (Fig 1B) implementing that in these cells upregulation of p21WAF1 occurred at transcriptional level. Since these cells show inactivation of p53 function22,25, it is likely that upregulation of p21WAF1 occurred either by p53-independent pathway or involves post-transcriptional mechanisms. We also performed p53 dependent reporter assays in NIH 3T3 and NIH 3T3/mot-2 cells. The activity of the full length p21WAF1 promoter reporter plasmid was three-five fold high in NIH 3T3 cells in different experiments22,25 and data not shown]. This suggested that the high level of p21WAF1 transcript in NIH 3T3/mot-2 cells is either regulated by elements located further upstream to the 2501 bp in the promoter used in the reporter plasmid or by post-transcriptional mechanism(s).
A. Western blot analysis with anti-p21WAF1 antibody in celllysates from normal (CMEF) cells at different passages (p), p3-p8 and immortal cells (RS-4, NIH 3T3 and NIH 3T3/mot-2) at different densities as indicated. A high level of p21WAF1 expression was detected in normal cells at later passages (senescence), in high density culture of NIH 3T3 cells and in low density culture of NIH 3T3/ mot-2 cells. Lower panel shows equal amount of protein loading as detected by probing of the same membrane with anti-actin antibody. B. Northern analysis for p21WAF1. A high level of p21WAF1 transcript was detected in NIH 3T3/mot-2 cells. The membrane was hybridized with 18S oligonucleotide probe for loading control. C. Western blot analysis of indicated cells with anti-p16INK4a antibody. An increase in p16INK4a was detected in CMEF during serial passaging. Similar to NIH 3T3, NIH 3T3/mot-2 cells lack the expression of p16 INK4a and served as a fingerprint for these cells.
Identity of the parent NIH 3T3 and its mot-2 derivative was affirmed by Western blot analysis with anti-p16INK4a antibody (Fig 1C). Consistent with the upregulation of p16INK4a during senescence of mouse and human cells9, 30, 31, 32, 33 we detected its high level of expression in late-passage mouse cells. And, consistent with the biallelic deletion of INK4a locus in NIH 3T3 cells34 p16INK4a was also not detected in either NIH 3T3 or NIH 3T3/mot-2 cells by Western blot analysis; confirming the identity of mot-2 derivatives to parent NIH 3T3 cells. RS-4 cells on the other hand have p16INK4aexpression and were nonmalignant in their growth properties. It has been shown that mice that carry targeted deletions of p16INK4a locus developed spontaneous tumors at an early age and are highly sensitive to carcinogenic treatment35. p16-/- mouse embryonic fibroblasts proliferate rapidly in culture, grow at high densities and show a high colony forming efficiency. An introduction of p16INK4a to human gliomas that undergo frequent loss of p16INK4a induces cellular senescence32. Such functions of p16INK4aand its presence in RS-4 cells may be related to their nonmalignant phenotype in spite of the presence of mutant p53 and other transformation favoring characteristics27, 36.
Taken together, these data presented evidence for post-transcriptional and p53-independent upregulation of p21WAF1 expression. Other examples of p53-independent increase in p21WAF1 expression include hepatocytes following carbon tetrachloride intoxication37, hematopoietic and hepatoma cells during differentiation38, human melanoma cells during IFN-b and MEZ induced growth arrest and terminal differentiation39, myeloid leukemic cells during terminal differentiation by a hormonal form of vitamin D40.
An amplification of p21WAF1 from NIH 3T3 and NIH 3T3/mot-2 cells by RT-PCR and sequence analysis (data not shown) of the amplified DNA product confirmed the wild type nature of p21WAF1 in malignantly transformed NIH 3T3/mot-2 cells. This indicated that an increased level of expression of p21WAF1 does not correlate with its cell cycle arrest function and may have some other function(s). In this context, elevated levels of p21WAF1 expression have been detected in gliomas41, non small cell lung carcinomas21,42, invasive cervical cancer43 and bladder carcinomas44. A 50-100 fold increase in p21WAF1expression has been detected in NIH 3T3 cells transformed with activated Ras oncogene20.
Cyclin D1 and cdk-2 are upregulated in stable NIH 3T3/mot-2 cells
An ectopic expression of cyclin D1 has been found to result in increased expression of p21WAF1 in human glioma and rodent fibroblasts that were morphologically transformed45. Such induction of p21WAF1 has been suggested to be mediated by E2F; an E2F binding site is found in p21Waf1 promoter[46]. A positive feed back regulation between cyclin D1 and p21WAF1 has been proposed. Cyclin D1/cdk-4 phosphorylate pRb and generate free E2F that can initiate p21WAF1transcription. Under the conditions when cyclin D1 levels are higher than p21WAF1, cells continue to divide and are unlikely to respond to p21-mediated growth inhibitory signals resulting into cellular transformation. Conversely, when p21WAF1 levels are greater than cyclin D1, cell cycle machinery is suppressed and/or shifted towards the direction of differentiation. Since NIH 3T3/mot-2 cells were seen to have a higher level of expression of p21WAF1 as compared to NIH 3T3 and CMEF cells, we next analyzed the expression of cyclin D1 in these cells (Fig 2A). A higher level of cyclin D1 was detected in NIH 3T3/mot-2 cells elucidating a factor that may positively affect p21WAF1 expression by abrogation of RB function through phosphorylation.
A. Western blot analysis for cyclin D1 in normal (CMEF), immortal (RS-4 and NIH 3T3) and malignantly transformed (NIH 3T3/mot-2) cells. An increase in expression level of cyclin D1 was detected in NIH 3T3/mot-2 cells. B. Western blot analysis of NIH 3T3 and its mot-2 derivative with anti-cdk-2, -4 and -6 antibodies. An increase in expression was detected for cdk-2 in mot-2 derivative. C. Western blot analysis of NIH 3T3 cells transiently transfected with GFP-tagged mot-1 and mot-2 proteins with anti-GFP, -cdk-2, -cyclin D1 and -actin antibodies. An equal level of expression was detected in control and transiently transfected cells.
Since active complexes of cyclin-cdks are essential for phosphorylation/ablation of tumor suppressor function of pRb for the release free E2F, we next analyzed expression profiles for cdk-2, cdk-4 and cdk-6 in NIH 3T3/mot-2 cells (Fig 2B). A low molecular mass band (the active form) of cdk-2 was detected at higher level in NIH 3T3/mot-2 as compared to NIH 3T3 cells. These data submit that such upregulated cdk-2 expression is likely to cooperate with cyclin D1 to phosphorylate RB and subsequent E2F-mediated increase in p21WAF1 expression in NIH 3T3/mot-2 cells as suggested above.
In contrast to the stable clones, transiently transfected cells showed decreased expression of p21WAF122. If the above described changes in cyclin D1 and cdk-2 are the antecedent for upregulated p21WAF1 expression, we reasoned that these changes should not be detected in transient transfectants. Indeed, in contrast to the stable clones, no change in the level of expression of cyclin D1 and cdk-2 was detected (Fig 2C) suggesting that upregulation of p21WAF1 in stable NIH 3T3/mot-2 cells may be mediated, at least in part, by upregulated cyclin D1 and cdk-2. The most likely mechanism of upregulated p21WAF1 expression appears to be arbitrated by active E2F, engendered by phosphorylation of RB that is caused by upregulated cyclin D1 and cdk-2. Mechanism of mot-2 interposed active cyclin D1 and cdk-2 levels in stable NIH 3T3/mot-2 cells and its absence in cells transiently transfected with plasmids encoding GFP-tagged mot-2 protein warrant further investigations.
In summary we have shown p53-independent upregulation of p21WAF1 in NIH 3T3 cells that are malignantly transformed by mot-2.
References
Levine AJ . p53, the cellular gatekeeper for growth and division. Cell 1997; 88:323–31.
Moll UM, Schramm LM . p53-an acrobat in tumorigenesis. Crit Rev Oral Biol Med 1998; 9:23–37.
Oren M, Prives C . p53: upstream, downstream and off stream. Biochim Biophys Acta 1996; 1288:R13–9.
Cinelli M, Magnelli L, Chiarugi, V . Redundant down-regulation pathways for p53. Pharmacol Res 1998; 37:83–5.
Almog N, Rotter V . An insight into the life of p53: a protein coping with many functions. Biochim Biophys Acta 1998; 1378:R43–54.
Prives C, Hall PA . The p53 pathway. J Pathol 1999; 187:112–26.
Gartel AL, Serfas MS, Tyner AL . p21-negative regulator of the cell cycle. Proc Soc Exp Biol Med 1996; 213:138–49.
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E . New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997; 11:847–62.
Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC . Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996; 93:13742–47.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW . Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593–602.
Zindy F, Quelle DE, Roussel MF, Sherr CJ . Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 1997; 15:203–211.
Weinberg RA . The molecular basis of carcinogenesis: understanding the cell cycle clock. Cytokines Mol Ther. 1996; 2:105–10.
Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR . Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 1994; 211:90–8.
Brown JP, Wei W, Sedivy JM . Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 1997; 277:831–4.
Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB . Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 1999; 18:4808–18.
Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA . p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 1999; 18:2789–97.
Reddel RR . Genes involved in the control of cellular proliferative potential. Ann N Y Acad Sci 1998; 854:8–19.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P . Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995; 82:675–84.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ . Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995; 377:552–7.
Michieli P, Li W, Lorenzi MV, Miki T, Zakut R, Givol D, Pierce JH . Inhibition of oncogene-mediated transformation by ectopic expression of p21Waf1 in NIH3T3 cells. Oncogene. 1996; 12:775–84.
Michieli P, Basilico C, Ando M, Givol D, Pierce J . (1997) Proc. AACR meeting (tumor suppressor genes) Abstract-B16. Wadhwa R, Takano S, Robert M, Yoshida A, Nomura H, Reddel RR, Mitsui Y, Kaul SC . Inactivation of tumor suppressor p53 by mot-2, a hsp70 family member. J Biol Chem 1998; 273:29586–91.
Kaul SC, Duncan EL, Englezou A, Takano S, Reddel RR, Mitsui Y, Wadhwa R . Malignant transformation of NIH3T3 cells by overexpression of mot-2 protein. Oncogene 1998; 17:907–11.
Wadhwa R, Kaul SC, Ikawa Y, Sugimoto Y . Identification of a novel member of mouse hsp70 family. Its association with cellular mortal phenotype. J Biol Chem 1993; 268:6615–21.
Wadhwa R, Takano S, Mitsui Y, Kaul SC . NIH 3T3 cells malignantly transformed by mot-2 show inactivation and cytoplasmic sequestration of the p53 protein. Cell Res 1999; 9:261–9.
Atadja P, Wong H, Garkavtsev I, Veillette C, Riabowol K . Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA 1995; 92:8348–52.
Kaul SC, Wadhwa R, Sugihara T, Obuchi K, Komatsu Y, Mitsui Y . Identification of genetic events involved in early steps of immortalization of mouse fibroblasts. Biochim Biophys Acta 1994; 1201:389–96.
Zeng YX, el-Deiry WS . Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene 1996; 12:1557–64.
Albrecht JH, Meyer AH, Hu MY . Regulation of cyclin-dependent kinase inhibitor p21(WAF1/Cip1/Sdi1) gene expression in hepatic regeneration. Hepatology 1997; 25:557–63.
Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G . Regulation of p16 CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 1996; 16:859–67.
Palmero I, McConnell B, Parry D, Brookes S, Hara E, Bates S, Jat P, Peters G . Accumulation of p16INK4a in mouse fibroblasts as a function of replicative senescence and not of retinoblastoma gene status. Oncogene 1997; 15:495–503.
Uhrbom L, Nister M, Westermark B . Induction of senescence in human malignant glioma cells by p16INK4A. Oncogene 1997; 15: 505–514.
Brenner AJ, Stampfer MR, Aldaz CM . Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 1998; 17:199–205.
Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ . Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell; 91:649–59.
Serrano M . The tumor suppressor protein p16INK4a. Exp Cell Res 1997; 237:7–13.
Sugihara T, Kaul SC, Mitsui Y, Wadhwa R . Enhanced expression of multiple forms of VEGF is associated with spontaneous immortalization of murine fibroblasts. Biochim Biophys Acta 1994; 1224:365–70.
Serfas MS, Goufman E, Feuerman MH, Gartel AL, Tyner AL . p53-independent induction of p21WAF1/CIP1 expression in pericentral hepatocytes following carbon tetrachloride intoxication. Cell Growth Differ 1997; 8:951–61.
Steinman RA, Hoffman B, Iro A, Guillouf C, Liebermann DA, el-Houseini ME . Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene 1994; 9:3389–96.
Jiang H, Lin J, Su ZZ, Herlyn M, Kerbel RS, Weissman BE, Welch DR, Fisher PB . The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene. 1995; 10:1855–64.
Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP . Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 1996; 10:142–53.
Jung JM, Bruner JM, Ruan S, Langford LA, Kyritsis AP, Kobayashi T, Levin VA, Zhang W . Increased levels of p21WAF1/Cip1 in human brain tumors. Oncogene 1995; 11:2021–8.
Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Bertacca G, Chella A, Merlo G, Angeletti CA, Dalla Palma P, Bevilacqua G . p21 RNA and protein expression in non-small cell lung carcinomas: evidence of p53-independent expression and association with tumoral differentiation. Oncogene. 1996; 12:1319–24.
Troncone G, Martinez JC, Palombini L, De Rosa G, Mugica C, Rodriguez JA, Zeppa P, Di Vizio D, Lucariello A, Piris MA . Immunohistochemical expression of mdm2 and p21WAF1 in invasive cervical cancer: correlation with p53 protein and high risk HPV infection. J Clin Pathol 1998; 51:754–60.
Zlotta AR, Noel JC, Fayt I, Drowart A, Van Vooren JP, Huygen K, Simon J, Schulman CC . Correlation and prognostic significance of p53, p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumors treated with bacillus Calmette-Guerin intravesical therapy. J Urol 1999; 161:792–8.
Hiyama H, Iavarone A, LaBaer J, Reeves SA . Regulated ectopic expression of cyclin D1 induces transcriptional activation of the cdk inhibitor p21 gene without altering cell cycle progression. Oncogene 1997; 14:2533–42.
Hiyama H, Iavarone A, Reeves SA . Regulation of the cdk inhibitor p21 gene during cell cycle progression is under the control of the transcription factor E2F. Oncogene 1998; 16:1513–23.
Acknowledgements
We thank Olivia M. Pereira- Smith, Baylor College of Medicine for mouse p21WAF1 cDNA. We also thank Tomoko Yaguchi for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
TAKANO, S., WADHWA, R., MITSUI, Y. et al. p53-independent upregulation of p21WAF1 in NIH 3T3 cells malignantly transformed by mot-2. Cell Res 11, 55–60 (2001). https://doi.org/10.1038/sj.cr.7290066
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290066