Abstract
Objective:
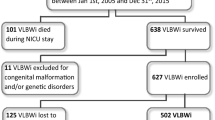
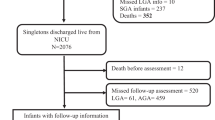
To report Bayley scores of 572 twenty-four-month corrected age infants whose birth weights (BWs) were less than 1500 g cared for in a Community Level 3 Neonatal Intensive Care Unit (NICU) between 1990 and 2002 when surfactant was routinely used.
Study design:
Survival, ‘normal’ defined as both Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI)>84, MDI>69 and MDI>84 were analyzed by gestational age (GA) and BW. Comparisons were made between infants born pre- and post-1996 when high-frequency oscillatory ventilation came into frequent use, Medicaid and non-Medicaid infants, multiples and singletons, outborn and inborn infants, boys and girls and infants with intrauterine growth retardation (IUGR) and those appropriate for gestational age (AGA).
Results:
There was a correlation between GA and BW and improving outcomes. Scores do not approach those of normal standardization sample populations (60% for ‘normal’, 68% for MDI>84 and 95% for MDI>69) until 1400 g and 30 weeks. Medicaid, outborn and IUGR infants, and boys did worse in some aspects.
Conclusion:
There was a correlation between both GA and BW and improving outcomes. Availability of these developmental data on a laminated pocket card can facilitate presentation of outcome experience to families by pediatric and obstetric caregivers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hallman M, Merritt A, Jarvenpaa AL, Boynton B, Mannino F, Gluck L et al. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr 1985; 106: 963–969.
Enhorning G, Shennan A, Possmayer F, Dunn M, Chen CP, Milligan J . Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics 1985; 76: 145–153.
Collaborative European Multicenter Study Group. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics 1988; 82: 683–691.
Fujiwara T, Konishi M, Shoichi C, Okuyama K, Ogawa Y, Takeuchi Y et al. Surfactant replacement therapy with a single postventilatory dose of reconstituted bovine surfactant in preterm neonates with respiratory distress syndrome: final analysis of a multicenter, double-blind, randomized trial and comparison with similar trials. Pediatrics 1990; 86: 753–764.
Bose C, Corbet A, Bose G, Garcia-Prats J, Lombardy L, Wold D et al. Improved outcome at 28 days of age for very low birth weight infants treated with a single dose of a synthetic surfactant. J Pediatr 1990; 117: 947–953.
Corbet A, Bucciarelli R, Goldman S, Mammel M, Wold D, Long W, and the American Exosurf Pediatric Study Group 1. Decreased mortality rate among small premature infants treated at birth with a single dose of synthetic surfactant: a multicenter controlled trial. J Pediatr 1991; 118: 277–284.
Hoekstra RE, Jackson JC, Myers TF, Frantz ID, Stern ME, Powers WK et al. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics 1991; 88: 10–18.
Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A et al. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics 1991; 88: 19–28.
Stevenson D, Walther F, Long W, Sell M, Pauly T, Gong A, et al., and the American Exosurf Neonatal Study Group I. Controlled trial of a single dose of synthetic surfactant at birth in Premature infants weighing 500–699 grams. J Pediatr 1992; 120: S3–S12.
Wilson-Costello D, Friedman H, Minich N, Fanaroff A, Hack M . Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 2005; 115: 997–1003.
Hack M, Wilson-Costello D, Friedman H, Taylor G, Schluchter M, Fanaroff A . Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med 2000; 157: 725–731.
Hack M, Taylor G, Drotar D, Schluchter M, Cartar L, Wilson-Costello D et al. Poor predictive validity of the Bayley scales of infant development for cognitive function of extremely low birth weight children at school age. Pediatrics 2005; 116: 333–341.
Koller H, Lawson K, Rose SA, Wallace I, McCarton C . Patterns of cognitive development in very low birth weight children during the first six years of life. Pediatrics 1997; 99: 383–389.
D'Angio C, Sinkin R, Stevens T, Landfish N, Merzbach J, Ryan R et al. Longitudinal, 15-year follow-up of children born at less than 29 weeks' gestation after introduction of surfactant therapy into a region: neurologic, cognitive, and educational outcomes. Pediatrics 2002; 110: 1094–1102.
Piecuch RE, Leonard CH, Cooper BA, Sehring SA . Outcome of extremely low birth weight infants (500 to 999 grams) over a 12-year period. Pediatrics 1997; 100: 633–639.
Marlow N, Wolke D, Bracewell MA, Sumara M, for the EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005; 352: 9–19.
Doyle LW, the Victorian Infant Collaborative Study Group. Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: I. Effectiveness. Pediatrics 2004; 113: 505–514.
Doyle LW, for the Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23–27 weeks' gestation: refining the prognosis. Pediatrics 2001; 108: 134–140.
Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD, for the National Institute of Child Health and Human Development Neonatal Research Network. Changes in neurodevelopmental outcomes at 18 to 22 months' corrected age among infants of less than 25 weeks' gestational age born in 1993–1999. Pediatrics 2005; 115: 1645–1650.
Laptook AR, O'Shea M, Shankaran S, Bhaskar B, the NICHD Neonatal Network. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics 2005; 115: 673–680.
Vohr BR, Wright LL, Dusick AM, Perritt R, Poole WK, Tyson JE, et al., for the Neonatal Research Network. Center differences and outcomes of extremely low birth weight infants. Pediatrics 2004; 113: 781–789.
Vohr BR, Wright LL, Poole WK, McDonald SA, for the NICHD Neonatal Research Network Follow-up Study. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005; 116: 635–643.
Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National institute of child health and human development neonatal research network, 1993–1994. Pediatrics 2000; 105: 1216–1226.
Bayley N . Bayley Scales of Infant Development, 2nd edn manual. 1993 p 216.
Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M . A United States national reference for fetal growth. Obstet Gynecol 1996; 87: 163–168.
Thomas P, Peabody J, Turnier V, Clark RH . A new look at intrauterine growth and the impact of race, altitude, and gender. Pediatrics 2000; 106: E21.
Fenton TR . A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr 2003; 3: 13–22.
Bayley N . Bayley Scales of Infant Development, 2nd edn manual. 1993 p 202.
Palmer FB . Strategies for the early diagnosis of cerebral palsy. J Pediatr 2004; 145: S8–S11.
Blanco F, Suresh G, Howard D, Soll RF . Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics 2005; 115: e478–e487.
Acknowledgements
We thank Dr Joan Hodgman for her extensive encouragement and reviews of the manuscript, Dr Maureen Hack for review of the data, Dr Richard Powers for review of the manuscript and Jie Zhang, MS, Stanford Department of Statistics, for her valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support or conflicts of interest: None.
Rights and permissions
About this article
Cite this article
Conom, D., Thomas, C., Evans, J. et al. Surfactant era (1990–2002) 2-year outcomes of infants less than 1500 g from a Community Level 3 Neonatal Intensive Care Unit. J Perinatol 26, 605–613 (2006). https://doi.org/10.1038/sj.jp.7211568
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211568
Keywords
This article is cited by
-
Extremely Preterm Birth Outcome: A Review of Four Decades of Cognitive Research
Neuropsychology Review (2010)