Abstract
Studies of fluctuating asymmetry (FA), a measure of developmental instability (DI), may provide insights into the importance of genetic factors in the long-term survival of small and isolated populations. In the present study of the rare, endemic plant Silene diclinis, I tested how moderate inbreeding within populations (full-sib crosses) and hybridization between populations influenced levels of developmental instability of petals and leaves, using plants derived from controlled crosses and raised under uniform growth conditions. The area and length of petals and leaves were digitized and measured with an image analysis system, but only bifid petals could be tested for true fluctuating asymmetry (normally distributed left-minus-right values with a mean of zero). Based on a bootstrap procedure, I found little evidence for directional asymmetry and antisymmetry in the area and length of the two lobes of the petals. Only the length measurements showed a significant leptokurtic distribution, which may reflect limited resolution (too few classes) in the image analysis system. Randomization tests were performed to test for differences between crossing treatments. Levels of FA of petal area and petal length were significantly higher for both the inbred and the interpopulation hybrid progenies relative to offspring from crosses between unrelated plants from the same population (control). There was no significant treatment effect on DI of leaves. Comparison of plants in the control group revealed that DI of leaf area was significantly higher than FA of petal area, and that these parameters were uncorrelated. This study demonstrates that FA of petals in Silene diclinis is sensitive to moderate levels of inbreeding and outbreeding, and therefore might serve as an indicator of genetic stress.
Similar content being viewed by others
Introduction
Developmental instability (DI), i.e. the inability of an organism to correct for random accidents under development (Waddington, 1957), has become a major topic in evolutionary biology. The failure to buffer the phenotype against random perturbations in bilateral organs may be manifested as fluctuating asymmetry (FA), defined as small, random deviations from bilateral symmetry (Palmer & Strobeck, 1986; Møller & Swaddle, 1997). Many studies have examined how FA responds to changes in genetic and environmental parameters. Although no clear patterns have been found, several trends have emerged. First, FA appears to increase with increasing homozygosity. Secondly, hybrids tend to be less developmentally stable than nonhybrids. Thirdly, individuals in extreme and stressful environments appear to have high levels of FA (Ross & Robertson, 1990; Palmer & Strobeck, 1992; Sherry & Lord, 1996a,b; Møller & Swaddle, 1997). Given these findings and the recent focus on inbreeding and outbreeding depression in conservation genetics (Frankham, 1995), studies of FA may provide valuable insights into the importance of genetic factors in the long-term survival of small, isolated populations (Leary & Allendorf, 1989; Clarke, 1995). There have been few attempts to quantify FA in populations of rare and threatened species (Clarke, 1995).
The estimation of FA is not without its problems (Palmer & Strobeck, 1992), particularly if the aim is to provide data on natural populations. One problem is to distinguish between genetic and environmental sources of FA. A number of investigations have estimated levels of heterozygosity of individuals (or populations) based on a small number of marker genes (e.g. allozymes) and then related this parameter to FA (reviewed in Mitton, 1993). This type of approach often has low statistical power and does not reveal the genetic basis of FA (Lynch & Walsh, 1997). Another common approach is to estimate the heritability of FA from data on related individuals (see Møller & Thornhill, 1997). Unfortunately, heritability estimates of FA can be expected to be severely biased (Whitlock, 1996). Moreover, from a theoretical basis it may be problematic to justify the heritability of FA when defined as a within-individual microenvironmental variance (see Gavrilets & Hastings, 1994). Instead, the genetic component of FA can be inferred by manipulating levels of inbreeding and outbreeding within and between populations by means of controlled crosses. In this approach, it would also be possible to minimize the macroenvironmental variance between individuals by estimating FA under controlled greenhouse or laboratory environments.
Different characters are often subjected to different selection pressures (Lynch & Walsh, 1997) and usually differ in patterns and amounts of FA (e.g. Evans & Marshall, 1996; Sherry & Lord, 1996a,b). Therefore, to identify suitable individuals (or populations) with low levels of genetic stress for conservation purposes, it is useful to know the level of association between FA of different characters. If levels of FA are uncorrelated, it may be better to focus on one or a few traits than to combine estimates of FA from widely different characters into an overall ‘quality index’. Possible candidates are characters that are correlated with fitness or respond to inbreeding and hybridization.
If the aim is to infer the genetic component of FA, it is also important to determine whether the data conform to ideal FA (normally distributed deviations from symmetry with a mean of zero) and to remove the effect of overall organ size before comparing levels of FA between different groups; for instance, distributions that depart from ideal FA provide unreliable estimates of levels of developmental instability if directional asymmetry or antisymmetry has a genetic basis (Palmer & Strobeck, 1992).
Prentice (1984) invoked inbreeding to explain the high incidence of developmental abnormalities in two natural populations of the threatened plant S. diclinis. In the present study, I examined how full-sib inbreeding and hybridization between populations influenced levels of FA of petals and levels of DI of leaves, using plants derived from controlled crosses and raised under uniform growth conditions. I also tested for differences between mean FA of petals and mean DI of leaves, and for correlations between: (i) petal FA and petal size; and (ii) petal FA and leaf DI. Computer image analysis was used to enhance the precision of the measurements, followed by randomization and bootstrap procedures to avoid the assumptions underlying parametric statistics.
Materials and methods
Silene diclinis (Lag.) Lainz (Caryophyllaceae) is a diploid, insect-pollinated, dioecious and perennial herb with a basal rosette and decumbent, branched stems up to 20 cm. The lanceolate leaves are opposite and each flower has five red, shallowly bifid petals. The species is confined to open patches within the matorral scrub and on the terraces of old and traditionally managed groves in a small region (15 × 6 km) near Xàtiva in the Spanish province of Valencia. The total number of individuals is less than 3000 (Prentice & Andersson, 1997). The present investigation is based on seed material (one capsule/female) collected in 1991–93 from spatially separated, open-pollinated females in the five remaining populations (referred to as LL, XAB, PS, QT and PC), which are separated by 2–17 km (Prentice & Andersson, 1997).
Following germination on moist filter papers in Petri dishes, a random sample of 12 seedlings from each sibship was planted in pots with standard soil and placed in random positions in an unheated greenhouse. Allozyme data based on eight polymorphic loci (Prentice & Andersson, 1997) were used to calculate the coefficient of relatedness (Queller & Goodnight, 1989) between individuals within offspring families, using FSTAT (Goudet, 1995). Progenies within full-sib families are expected to have a relatedness coefficient of 0.5 and progenies within half-sib families a value of 0.25 (Queller & Goodnight, 1989). The jackknifed estimate of the relatedness coefficient in the present study was 0.513 (SE 0.033), showing that the majority of individuals within families were full-sibs. When a majority of the plants had reached anthesis, different flowers on each of 16 females from different populations (two from QT; three from PC, PA and XAB; five from LL) were mated to randomly selected males from the following categories (treatments): (i) the same maternal family (full-sib inbreeding); (ii) another family in the same population (control); and (iii) a family representing another population (population hybrid).
In the following year (1996), I planted 10 seedlings per cross in separate pots, randomized the pots in the greenhouse and pressed three pairs of opposite leaves and three petals from each plant. Detailed measurements were obtained from two plants per family × treatment combination (n = 269 petals, 270 leaf pairs).
I used computer-assisted image analysis techniques (IMAGEGRABBERTM, OPTILABTM) to measure the length and area of the two leaves in each ‘leaf pair’ and the two lobes of each petal. The symmetry axis of a petal was defined as the line connecting the indentation at the distal part and the midpoint at the transition between the limb and claw (Fig 1). The deviation from symmetry in length and area was estimated as the difference between the right and the left lobes standardized by the average of these measures (hereafter RL), whereas FA was estimated as the absolute values of RL (index no. 2 of Palmer & Strobeck, 1986). Unfortunately, it was not possible to assign a bilateral symmetry axis with a repeatable precision to individual leaves. Instead, I used the absolute difference between the length and the area of the two opposite leaves in each leaf pair, divided by their average, to quantify levels of instability in leaf development (hereafter leaf DI). Area was used as an asymmetry measure under the assumption that the shape of the objects was reasonably constant.
To test for directional asymmetry, I employed a bootstrap procedure (Manly, 1997, p. 39) with 5000 random samples from the original dataset to construct percentile confidence intervals (CI) of the averaged RL values. The null hypothesis of symmetry was rejected if this CI excluded 0. A similar approach was used to test for skewness and kurtosis, quantified by the moment statistics g1 and g2 (Sokal & Rohlf, 1995). Significant deviations from zero would indicate skewness to the left (negative g1), skewness to the right (positive g1), platykurtosis (negative g2) and/or leptokurtosis (positive g2). Bootstrap analyses were also used to obtain point estimates and standard errors of petal FA and leaf DI for each treatment. All these procedures considered petals and leaf pairs as statistically independent observations.
To quantify the variance arising from measurement error, I determined the RL value for each of 20 repeated measurements of one petal and calculated the variance of these values. This estimate (Verror) was compared with the variance of RL values in the control group (Vcontrol) by calculating the ratio (Verror/Vcontrol) for each of 5000 pairs of bootstrap samples (one from each dataset). A ratio significantly smaller than 1 would indicate that measurement error accounts for only a minor fraction of the RL variance, particularly if the value is close to 0. The analysis of measurement area was restricted to the floral data; the round shape of the petals makes these structures more prone to measurement error than the larger and relatively narrower leaves.
I performed robust one-sided randomization procedures (Manly, 1997, p. 6) with 10 000 permutations to test for: (i) differences in petal FA and leaf DI between treatments; (ii) differences between petal FA and leaf DI in the control group; and (iii) inbreeding/outbreeding depression and heterosis of petal area. The randomization procedure allowed me to support a specific alternative hypothesis (e.g. FAInbred > FAControl) upon rejection of the null hypothesis. There was no difference between the sexes in FA of petal area (randomization test: P = 0.240), so females and males were pooled in all analyses. Given the small number of families available, no attempt was made to compare FA and DI of different populations. To test for an association between petal FA and leaf DI, and between petal FA and petal area (sum of both lobes) in the control group, I computed the Pearson correlation coefficient (r) based on means for each individual and estimated the CI of r from 5000 bootstrap samples. All analyses were carried out using GENSTAT 5.3 (1996).
Results
Judging from the 95% CI, the bootstrapped means of the RL scores for petal area and petal length were not significantly different from 0 for any of the treatment groups. The skewness parameter (g1) was negative in most cases and significantly smaller than zero for RL of petal area in the inbred group, whereas the corresponding estimate for petal length failed to reach significance (Table 1). Neither petal area nor petal length had a statistically significant value of g1 in the control and hybrid groups. Hence, there was little evidence for directional asymmetry in petal morphology. No significant kurtosis was detected for the RL scores of petal area. Conversely, RL of petal length showed positive kurtosis in all treatment groups (Table 1), suggesting a narrower peak than expected for a normal distribution (leptokurtosis). None of the distributions showed evidence of platykurtosis, i.e. there was no antisymmetry (Table 1). Measurement error accounted for only a minor fraction of the variance of the RL scores, the ratio (Verror/Vcontrol) being higher for petal length (0.0298, 100% CI: 0.0001–0.0912) than for petal area (0.0045, 100% CI: 0.0014–0.0099).
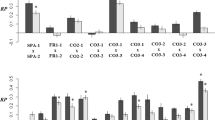
The FA of both petal area and petal length was significantly higher for inbred progenies than for plants representing the control group (Fig 2, Table 2). Moreover, between-population hybrids had higher FA than the control plants in both variables (Fig 2, Table 2). The level of DI in leaf area and leaf length differed little between treatment groups (Fig 2), except for the weakly significant difference between hybrid progenies and control plants (leaf length; Table 2). There was no evidence for inbreeding depression in petal area (inbred vs. control plants; P = 0.371), whereas the population hybrids showed a significant heterosis effect (mean petal area of hybrids 2.98 mm2 larger than the mean petal area of control plants; P = 0.0324).
Analysis of data in the control group revealed significantly (P = 0) larger deviations from symmetry for leaf area and leaf length (leaf DI = 0.146 and 0.0771, respectively) than for petal area (FA = 0.0665) and petal length (FA = 0.0115). There was no correlation between petal FA and leaf DI (r = 0.048, 95% CI: −0.190–0.303). Finally, there was a tendency for FA of petal area to decrease with increasing petal area, but this correlation was too weak to be declared as significant (r = −0.151; 95% CI: −0.351–0.054).
Discussion
The present investigation of the rare, endemic plant S. diclinis demonstrates that inbreeding and hybridization between populations have negative effects on developmental stability, at least in floral morphology (petal length and area). The finding that increased inbreeding disrupts developmental stability is consistent with previous attempts to link developmental stability with homozygosity in S. diclinis (Prentice, 1984), and agrees with the relationship between levels of inbreeding and FA documented in several previous studies of plants (Levin, 1970; Sherry & Lord, 1996a,b) and animals (Palmer & Strobeck, 1986). The present study also complements the work of other investigators who demonstrated higher FA in intra- and interspecific crosses, than in nonhybrids (e.g. Ross & Robertson, 1990; see also Leary & Allendorf, 1989).
I found little evidence for directional asymmetry and antisymmetry in the RL scores for area and length of the two lobes of the petals, the only exception being a slight (but significant) negative skewness for petal area of inbred plants. This deviation from normality is difficult to explain, particularly as the mean deviation from symmetry was close to 0. According to Palmer & Strobeck (1992), such patterns can arise when the sample is a mixture of observations from groups with different patterns of asymmetry. Whether this explanation also applies to the present study of S. diclinis depends on the extent to which families from different populations responded differently to inbreeding.
All treatment groups showed a leptokurtic distribution of RL scores for petal length. Such deviations from normality can arise in several ways (Palmer & Strobeck, 1992). One possibility is that the leptokurtic distributions represent the effect of pooling samples that exhibit different kinds of asymmetry, or samples that exhibit different levels of ‘true’ FA. Another explanation is that they arise as an allometric effect during development. However, given the more or less normal distribution of the RL scores for petal area (and the low measurement error variance estimated for this variable), I suggest that the leptokurtic distribution in petal length may be a result of limited resolution in the image analyses (≈ 0.2 mm/pixel on objects that were approximately 1 cm long). This precision is comparable with that used when measuring objects with a micrometer under a microscope (P. Waldmann, pers. obs.), suggesting that analyses of FA in linear dimensions with small ranges should be interpreted with care (see also Swain, 1987).
Inbreeding and outbreeding may influence developmental stability through heterozygote advantage (overdominance), through the expression of recessive genes, and through the break-up of coadapted gene complexes (Markow, 1995). The present investigation was not designed to elucidate the genetic mechanism behind developmental stability, but the results provide a few observations that may bear on this issue. First, inbreeding had a much greater effect on petal FA than on petal size, and there was no significant linear (i.e. additive) relationship between these variables (see Gavrilets & Hastings, 1994). Hence, there is reason to believe that FA and trait value are controlled by different genes, as suggested by Waddington (1957). Secondly, the finding of heterosis in the interpopulation hybrids does not exclude hybrid breakdown in subsequent generations (Lynch & Walsh, 1997). Outbreeding depression has been observed in several plant species that show adaptive divergence over very short distances (Waser, 1993). Therefore, break-up of coadapted gene complexes (favourable epistatic interactions) could still account for the increased FA after hybridization, but more detailed genetic data are needed to test this hypothesis.
The crossing treatments had little influence on leaf DI and there was no correlation between estimates of leaf DI and petal FA of plants in the control group. That different types of character differ in patterns and amounts of developmental stability has been demonstrated in many studies (e.g. Møller & Eriksson, 1994; Sherry & Lord, 1996a,b). Based upon these observations, there seems to be no reason for calculating an overall index of DI (or FA) based on several unrelated characters to identify populations subjected to genetic stress. Instead, it would be preferable to focus on specific characters that show a relationship between FA, genetic variation and/or fitness. More data are needed to examine the relationship between FA in floral traits and direct components of fitness in S. diclinis. There is some evidence from other species that large, symmetrical flowers attract more pollinators than small, asymmetric flowers (Møller & Sorci, 1998), but further studies are required to determine whether FA or size represents the most important cue for pollinators.
The tendency for floral organs to be more developmentally stable than leaves in S. diclinis has been found in several other plant species (Møller & Eriksson, 1994; Evans & Marshall, 1996; Sherry & Lord, 1996a,b). This is consistent with the generalization that vegetative characters tend to be more environmentally plastic (less canalized) than floral traits (Bradshaw, 1965). It is possible that opposite leaves experience slightly different light conditions and that a plastic response to these differences biased the estimate of leaf DI upwards. To the extent that FA in floral characters is a reliable indicator of genetic stress, future studies should focus on the relationship between developmental stability of flowers and different aspects of fitness. Furthermore, for FA to be a useful tool in the monitoring of threatened species, it is necessary to demonstrate that an increased level of developmental stability occurs before detrimental effects of inbreeding and outbreeding come into play. This study shows that moderate genetic perturbations can induce significant levels of developmental instability.
References
Bradshaw, A. D. (1965). Evolutionary significance of phenotypic plasticity in plants. Adv Genet, 13: 115–155.
Clarke, G. M. (1995). Relationships between developmental stability and fitness: application for conservation biology. Conserv Biol, 9: 18–24.
Evans, A. S. and Marshall, M. (1996). Developmental instability in Brassica campestris (Cruciferae): fluctuating asymmetry of foliar and floral traits. J Evol Biol, 9: 717–736.
Frankham, R. (1995). Conservation genetics. Ann Rev Genet, 29: 305–327.
Gavrilets, S. and Hastings, A. (1994). A quantitative-genetic model for selection on developmental noise. Evolution, 48: 1478–1486.
GENSTAT 5 (1996). Release 3.2. Lawes Agricultural Trust. Rothamsted Experimental Station, Rothamsted, U.K..
Goudet, J. (1995). FSTAT V-1.2: a computer program to calculate F-statistics. J Hered, 86: 485–486.
Leary, R. F. and Allendorf, F. W. (1989). Fluctuating asymmetry as an indicator of stress: implications for conservation biology. Trends Ecol Evol, 4: 214–217.
Levin, D. A. (1970). Developmental stability and evolution in peripheral isolates. Am Nat, 104: 343–353.
Lynch, M. and Walsh, B. (1997). Genetics and Analysis of Quantitative Traits. Sinauer Associates, Sunderland, MA.
Manly, B. F. J. (1997). Randomization, Bootstrap and Monte Carlo Methods in Biology 2nd edn., Chapman & Hall, New York.
Markow, T. A. (1995). Evolutionary ecology and developmental instability. Ann Rev Ent, 40: 105–120.
Mitton, J. B. (1993). Enzyme heterozygosity, metabolism, and developmental stability. Genetica, 89: 47–65.
Møller, A. P. and Eriksson, M. (1994). Patterns of fluctuating asymmetry in flowers: Implications for sexual selection in plants. J Evol Biol, 7: 97–113.
Møller, A. P. and Sorci, G. (1998). Insect preference for symmetrical artificial flowers. Oecologia, 114: 37–42.
Møller, A. P. and Swaddle, J. P. (1997). Asymmetry, Developmental Stability, and Evolution. Oxford University Press, Oxford.
Møller, A. P. and Thornhill, R. (1997). A meta-analysis of the heritability of developmental stability. J Evol Biol, 10: 1–16.
Palmer, A. R. and Strobeck, C. (1986). Fluctuating asymmetry: Measurement, analysis, patterns. Ann Rev Ecol Syst, 17: 391–421.
Palmer, A. R. and Strobeck, C. (1992). Fluctuating asymmetry as a measure of developmental stability: implications of non-normal distributions and power of statistical tests. Acta Zool Fenn, 191: 57–72.
Prentice, H. C. (1984). Enzyme polymorphism, morphometric variation and population structure in a restricted endemic, Silene diclinis (Caryophyllaceae). Biol J Linn Soc, 22: 125–147.
Prentice, H. C. and Andersson, S. (1997). Genetic variation and population size in the rare dioecious plant, Silene diclinis (Caryophyllaceae). In: Tew, T. E., Crawford, T. J., Spencer, J. W., Stevens, D. P., Usher, M. B. and Warren, J. (eds) The Role of Genetics in Conserving Small Populations, pp. 65–72. JNCC, Peterborough.
Queller, D. C. and Goodnight, K. F. (1989). Estimating relatedness using genetic markers. Evolution, 43: 258–275.
Ross, K. G. and Robertson, J. L. (1990). Developmental stability, heterozygosity, and fitness in two introduced fire ants (Solenopsis invicta and S. richteri) and their hybrid. Heredity, 64: 93–103.
Sherry, R. A. and Lord, E. M. (1996a). Developmental stability in flowers of Clarkia tembloriensis (Onagraceae). J Evol Biol, 9: 911–930.
Sherry, R. A. and Lord, E. M. (1996b). Developmental stability in leaves of Clarkia tembloriensis (Onagraceae) as related to population outcrossing rates and heterozygosity. Evolution, 50: 80–91.
Sokal, R. R. and Rohlf, F. J. (1995). Biometry. 3rd edn. W. H. Freeman, New York.
Swain, D. P. (1987). A problem with the use of meristic characters to estimate developmental stability. Am Nat, 129: 761–768.
Waddington, C. H. (1957). The Strategy of the Genes. Macmillan, New York.
Waser, N. M. (1993). Population structure, optimal outbreeding, and assortative mating in Angiosperms. In: Thornhill, N.W. (ed.) The Natural History of Inbreeding and Outbreeding pp. 173–199. Chicago University Press, Chicago.
Whitlock, M. (1996). The heritability of fluctuating asymmetry and the genetic control of developmental stability. Proc R Soc B, 263: 849–854.
Acknowledgements
Many thanks to Stefan Andersson and Honor C. Prentice for providing material and for discussions; with Richard Palmer they provided useful comments on the manuscript. The study was partly funded by a grant from the Swedish Natural Science Research Council (to H.C. Prentice) and by a grant from Anna och Svante Murbecks minnesfond to the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldmann, P. The effect of inbreeding and population hybridization on developmental instability in petals and leaves of the rare plant Silene diclinis (Caryophyllaceae). Heredity 83, 138–144 (1999). https://doi.org/10.1046/j.1365-2540.1999.00545.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.1999.00545.x
Keywords
This article is cited by
-
Outbreeding causes developmental instability in Drosophila subobscura
Evolutionary Ecology (2010)
-
The effect of inbreeding on fluctuating asymmetry of wing veins in two laboratory strains of Drosophila melanogaster
Heredity (2009)
-
Effect of directional selection for body size on fluctuating asymmetry in certain morphological traits in Drosophila ananassae
Journal of Biosciences (2009)
-
Developmental stability in Brassica cretica: the effect of crossing distance on fluctuating asymmetry in cotyledon morphology
Heredity (2002)