Abstract
Seven different mildew resistant wheat lines derived from crosses between triticale and bread wheat were examined by molecular cytogenetics and chromosome C-banding in order to determine their chromosomal composition. Genomic in situ hybridisation (GISH) showed the presence of rye germplasm in all the lines and identified three substitution lines, three double substitution lines and one addition-substitution line. C-banding identified rye chromosomes 1R and 4R in the addition-substitution line, rye chromosomes 1R and 6R in two substitution lines and 1R and 2R in the third line, and rye chromosome 1R in the three substitution lines. Two of the latter lines (7–102 and 7–169) contained a modified form of the chromosome; fluorescent in situ hybridisation (FISH) using five different repetitive DNA-probes showed a pericentric inversion of 1R in both lines. The breakpoints of the 1R inversion were between (1) the 5S rDNA site and the NOR-region on the satellite of the short arm, and (2) between two AAC(5) sites close to the centromere on the long arm. The role of the rye chromosomes in the mildew resistance, the utilisation of the inverted 1R and the significance of the lines in wheat breeding are discussed.
Similar content being viewed by others
Introduction
The rye (Secale cereale L.) genome has shown potential for improvement of bread wheat (Triticum aestivum L.), where wheat-rye substitutions and translocations have been and are frequently used in resistance breeding (Rabinovich, 1998) and the 1B/1R wheat-rye translocation is present in the highest yielding cultivars currently grown in Europe and Canada (Heslop-Harrison et al, 1990). A detailed analysis of the introgressed rye chromatin is essential for practical breeding. Molecular cytogenetics has proven to be an efficient method to determine the origin of genomes, chromosomes and parental genomes in hybrids (Schwarzacher et al, 1989). The advantage of genomic in situ hybridisation (GISH) is the recognition of all alien chromosome segments contained in the nucleus and is therefore the method of choice when interspecific crosses and derived introgressed lines are analysed to reveal alien chromosomes and translocations (Murata et al, 1992; Schwarzacher et al, 1992; Mukai et al, 1993; Taketa et al, 1997).
Fluorescent in situ hybridisation (FISH) enables physical mapping of repetitive DNA sequences to specific chromosomal sites. In both wheat and rye several such probes are cloned and can be used for identification of chromosomes in those genomes and their chromosomes (Mukai et al, 1993; Castilho and Heslop-Harrison, 1995; Cuadrado et al, 1995, 2000; Pedersen and Langridge, 1997; Cuadrado and Schwarzacher, 1998). Physical mapping of differentially labelled repetitive DNA sequences simultaneously in one experiment and in combination with total genomic DNA (eg Leitch et al, 1991; Mukai et al, 1993; Taketa, 1997) allows introgressed chromosomes or chromosome segments from alien species to be detected in wheat lines, identifying the chromosomes involved and determining the nature and organisation of any chromosome rearrangements.
This study was performed in order to identify and characterise rye chromosomes that were detected in mildew resistant wheat lines isolated in offsprings from triticale × wheat hybrids. GISH, DAPI staining and C-banding was used to identify rye chromatin in the substitution and addition-substitution lines and FISH with repetitive probes to identify most wheat chromosomes and fine mapping the rearrangement detected in an aberrant chromosome found in two of the lines.
Materials and methods
Plant material
In populations derived from crosses between hexaploid triticale and bread wheat, lines with a broad mildew resistance were isolated (Forsström and Merker, 2001). Briefly, hexaploid winter triticales were crossed and backcrossed to mildew susceptible wheat cultivars, and then selfed. Screening for mildew resistance was performed by using three different mildew isolates. Seven lines were analysed in the present study: 1–17 in F7 and 7–73, 7–102, 7–169, 7–225, 7–317a and 7–317b in BC1F6 showed resistance to all isolates and all of them were fully fertile. These lines are maintained in Sweden at the Department of Crop Science in Alnarp at the Swedish University of Agricultural Sciences.
C-banding
Chromosome C-banding followed the method described by Gill et al (1991) and used excised root tips pre-treated with 0.05% colchicine for 3 h in room temperature followed by 24 h in ice water before fixation in 3:1 (v/v) ethanol:acetic acid.
Preparation of chromosomes for in situ hybridisation
Seed germination and chromosome preparation followed the technique as described by Schwarzacher et al (1989) and Schwarzacher and Heslop-Harrison (2000). Seedling roots were synchronised with 24-h ice treatment before fixation, the cell wall material was digested with 3% (w/v) pectinase (Sigma, 450 u/ml), 1.8% (w/v) cellulase (Calbiochem, 4000 u/g) and 0.2% (w/v) cellulase (Onozuka RS, 5000 u/g) and chromosome preparations made on glass slides by squashing in 45% and 60% acetic acid.
Probes and labelling
The following probes were used in this study: pTa71 contains a 9-kb EcoRI fragment of the repeat unit of 25S-5.8S-18S rDNA isolated from T. aestivum (Gerlach and Bedbrock, 1979) and re-cloned in pUC19. The clone was linearised with HindIII before labelling. pTa794 contains a 410 bp BamHI fragment of the 5S rDNA isolated from T. aestivum (Gerlach and Dyer, 1980). pSc119.2 contains a 120 bp tandem repeated DNA sequence isolated from S. cereale and subcloned by McIntyre et al (1990). pSc200 contains a 380 bp tandem repeat sequence from S. cereale cloned in pUC18 (Vershinin et al, 1995). dpTa1 contains a tandem repeat with a monomeric length of 340 bp isolated from T. aestivum and subcloned by Vershinin et al (1994). This probe is D-genomic and homologous to pAs1 (Rayburn and Gill, 1986). AAC(5) is a synthetic oligonucleotide sequence (Genosys). Total genomic DNA rye ‘Petkus’ was sheared to 5–8 kb pieces by autoclaving. For labelling, biotin-16-dUTP and digoxigenin-16-dUTP (Roche Diagnostics) were incorporated in separate reactions. pTa71 and total genomic rye DNA were labelled by nick translation. pSc119.2, pSc200, pTa794 and dpTa1 were labelled by PCR using the universal forward and reverse M13 sequencing primers. AAC(5) was labelled by random priming (Cuadrado et al, 2000).
In situ hybridisation
Probe mixture, DNA:DNA in situ hybridisation and detection followed the method described by Schwarzacher and Heslop-Harrison (2000). The probe mixture contained 50% (v/v) formamide, 20% (w/v) dextran sulphate, 2 × SSC, 25–100 ng probe, 20 μg of salmon sperm DNA and 0.3% sodium dodecyl sulfate (SDS), and in some experiments autoclaved total genomic DNA from wheat cv. ‘Chinese Spring’ as blocking DNA at 4–20 × probe concentration. Hybridisation took place over night at 37°C and the most stringent wash was carried out with 20% (v/v) formamide and 0.1 × SSC at 42°C. Hybridisation sites were detected with 2.0 μg/ml streptavidin conjugated to Alexa594 (Molecular Probes) or 3.0 μg/ml streptavidin conjugated to Cy3 (Sigma) and 4 μg/ml antidigoxigenin conjugated to flourescein isothiocyanate (FITC) (Roche Diagnostics). Chromosomes were counterstained with 0.2 μg/ml DAPI (4′,6-diamidino-2-phenylindole) diluted in McIlvaines buffer pH7 and mounted in antifade solution (Citiflour).
Photography and analysis
Photographs of C-banding were taken with Kodak technical pan black and white film, and in situ hybridisation with Fujicolor SuperHG400 colour film. Negatives were scanned to CD-rom by Kodak Digital Science. Colour figures were prepared using Adobe Photoshop (Adobe Systems) with only those processing functions that are equally applied to all pixels of the image.
For each line, 15 metaphases were analysed after Giemsa C-banding following the karyotypes published by Sybenga (1983) and Gill et al (1991), 10 metaphases after DAPI staining and in at least four complete and partial metaphases for each of the genomic rye and repetitive in situ hybridisation probes. Detailed signal of the derivative 1R (der1R) was analysed in a total of 50 individual chromosomes hybridised with various probe combinations and the combined signals superimposed on the published chromosome morphology of chromosome 1R (Cuadrado et al, 1995) in Figure 3.
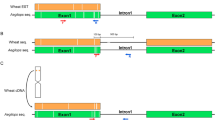
Schematic diagram of normal chromosome 1R and chromosome der1R and proposed breakpoints of the pericentric inversions. Chromosome morphology and band distribution was taken from Cuadrado et al (1995), Castilho and Heslop-Harrison (1995) and Cuadrado and Schwarzacher (1998).
Results
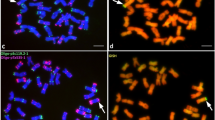
C-banding of the progeny of a cross between a mildew resistant triticale line and susceptible bread wheat, identified one addition-substitution line containing rye chromosomes 1R and 4R, three double wheat-rye substitution lines containing rye chromosomes 1R and 6R (Figure 1a) or chromosomes 1R and 2R, a wheat-rye substitution line with the presence of rye chromosome 1R and two wheat-rye substitution lines with der1R (Figure 1b). GISH (Table 1 and Figure 2b, f) showed the presence of rye chromatin in the six investigated lines and FISH with repetitive probes was used in five of the lines (Table 1 and Figure 2d, g–n), which gave specific information about the identity of chromosomes and chromosome segments. The results are summarised in Table 1 and are briefly described below.
Photomicrographs of DAPI staining and fluorescent in situ hybridisation with total genomic rye DNA and cloned or synthetic probes to metaphases of mildew resistant wheat lines incorporating rye chromatin. (a, b) Line 7–73 contains rye chromosome pairs 1R and 6R that show subtelomeric bands with DAPI (a) and are labelled strongly with total genomic DNA from rye (b). (c, d) Line 7–225: after DAPI staining chromosome 1R can be identified by its three heterochromatic bands. Probes pTa71 and dpTa1, both labelled with digoxigenin and detected with yellow-green fluorescence, identified the larger NOR bands on chromosomes 1R, 1B and 6B (arrows); and 12 D-genome chromosomes with several small interstitial bands (arrow heads). (e–g) Line 7–102: the same metaphase probed with rye genomic DNA (red in f and g) and pTa71 (yellow-green in g) and stained with DAPI (blue in e). The der chromosome 1R has a very unequal arm ratio and is characterised by a large pTa71 site in the long arm. Eight further sites of pTa71 are detected on the wheat chromosomes (arrows in g). (h) Line 7–102: the probe (AAC)5 hybridises to all B genome chromosomes, chromosome 4A and identifies four significant bands on der1R. (i–j) Line 7–102: the same der1R chromosome hybridised with pSc200 (yellow in i), (AAC)5 (red in j) and stained with DAPI (blue in k). The overlay in (l) shows the relation of the probes and DAPI bands. (m) Line 7–102: chromosome der1R probed with pTa71 (yellow) and (AAC)5 (red) and stained with DAPI (blue). (n) Line 7–102: chromosome der1R probed with pTa71 (yellow) and pSc119.2 (red) and stained with DAPI (blue). Bar equals 12 μm in a–d and h; 10 μm in e–g and 17.5 μm in i–n.
Line 1–17
Line 1–17 was an addition-substitution line (2n = 44) with the presence of the rye chromosomes 1R and 4R as detected by C-banding. GISH confirmed the presence of four rye chromosomes and DAPI-staining the presence of 1R. This line had small seeds and was relatively weak.
Line 7–73
Line 7–73 was a double substitution line (2n = 42) and the rye chromosomes 1R and 6R were identified both with C-banding and DAPI (Figure 2a). The presence of four rye chromosomes was also detected by GISH (Figure 2b). The NORs were identified by the probe pTa71 and revealed the presence of 1R, 1B, 6B, 1A and 5D (Table 1). The hybridisation pattern with probe pSc119.2 confirmed the presence of 6R. The probe dpTa1 revealed that the line lacked four D-chromosomes. It is most likely that the line had the chromosomal composition 1R(1D) and 6R(6D).
Line 7–317a
Line 7–317a was a substitution line (2n = 42) and C-banding revealed the presence of rye chromosomes 1R and 2R (Table 1). This line was not analysed by in situ hybridisation.
Line 7–317b
Line 7–317b was a substitution line (2n = 42) and C-banding identified the rye chromosomes 1R and 6R, while GISH showed the presence of four rye chromosomes. Hence this line (7–317) consisted of two different sub lines, and the 6R sub line, 7–317b, was studied with FISH. The probe pTa71 detected the presence of 1R, 1B, 6B, 1A and 5D. The probe dpTa1 identified the presence of 12 D-genome chromosomes. The rye chromosome 1R had substituted 1D while 6R had substituted 6A.
Line 7–225
Line 7–225 was a substitution line (2n = 42) and rye chromosome 1R was identified by C-banding and DAPI-staining (Figure 2c). GISH confirmed the presence of two rye chromosomes. Probe pTa71 identified six major NORs and four minor NORs, which indicated the presence of 1R, 1B, 6B and 1A, 5D (Table 1). The D-genome specific probe dpTa1 showed the presence of 12 D-chromosomes (Figure 2d). We concluded that 1D was replaced by 1R in a 1R(1D) substitution.
Line 7–102
This line was a substitution line (2n = 42) as determined by C-banding (Figure 1b) and DAPI-staining (Figure 2e). C-banding showed an unusual chromosome pair with subtelomeric bands and a strong heterochromatic band 1/3 of the way down the long arm (Figure 1b). This latter band was similar in size with the NOR of the short arm of the normal 1R (compare Figure 1b with Figure 1a). GISH showed that entirely rye chromatin was contained in the unusual chromosome (Figure 2f) and no other translocation either with wheat or rye chromosomes seemed to have contributed to the rearrangement.
In situ hybridisation with pTa71 confirmed the presence of the NOR in the intercalary position of the long arm, identifying the aberrant chromosome as a der1R. pTa71 also showed the four major and the four minor NORs of the wheat chromosomes 1B, 6B, 1A and 5D. The probe dpTa1 determined the presence of 12 D-genome chromosomes (Table 1) supporting the presence of a 1R(1D) substitution. Probe pSc200 hybridises to both subtelomeric regions of 1R (Vershinin et al, 1995) and it hybridised at the same positions in der1R (Figure 2i). Similarly, the location of the 5S rDNA as detected with the probe pTa794 was unaltered at the distal end of the short arm (Figure 3). The probe pSc119.2 hybridised at both subterminal regions and at an intercalary position in both arms of the normal 1R (Cuadrado et al., 1995). Both subterminal bands and the intercalary band of the long arm were detected at normal sites in der1R, while a second intercalary band was found in the long arm, proximal to the NOR (Figure 2n and 3). The probe AAC(5) detected paracentromeric sites on both chromosome arms of the normal 1R, the one of the long arm being slightly stronger than the one on the short arm, in addition to a proximal intercalary and a subtelomeric site on the long arm (Cuadrado and Schwarzacher, 1998). The subtelomeric site was located proximally to the pSc119.2 site and was found in the same position in the der1R chromosome (Figures 2h–m and 3). The paracentromeric sites were rearranged, the one on the short arm now being stronger (eg Figure 2j) and the interstitial site was distally located relative to the NOR-region.
Line 7–169
This line was a substitution line (2n = 42) as determined by C-banding (Table 1) containing the aberrant chromosome der1R. Line 7–169 seemed to be identical to line 7–102 (compare lines 7–102 and 7–169 in Table 1), as far as the rye chromosomes was concerned, which could be derived from a single meiotic event.
Chromosome der1R
From interpretation of all the FISH results we concluded that the der1R is a pericentric inversion with the breakpoints (1) between the 5S rDNA and the 45S rDNA site on the short arm and (2) between the paracentromeric and proximal interstitial site of AAC(5) on the long arm, transferring the NOR-region and a pSc119.2 site to the long arm that now is 75% of the chromosome (Figure 3). From comparing the overall lengths of the chromosome, the size of hybridisation signals and distances between the different sites it is most likely that no 1R chromatin material was lost during the rearrangement and that no material from wheat or other rye chromosomes was incorporated.
Discussion
The present study using C-banding (Figure 1) and FISH (Figure 2) has shown the disomic presence of rye chromosomes in all the investigated wheat lines. Rye chromosome 1R was found in all the lines, as the only rye chromosome in three lines and together with either of the rye chromosomes 2R, 4R or 6R in the other lines (Table 1). Use of repetitive probes to identify most of the wheat chromosomes allowed us to determine that chromosome 1R replaced chromosome 1D in all investigated lines, that chromosome 6R replaces 6A in one and 6D in another line, and that chromosome 4R is present as an addition.
Rye chromosomes 1, 2, 4 and 6 are known carriers of mildew resistance (Driscoll and Jensen, 1965; Zeller, 1973; Lind, 1982; Heun et al, 1990; Friebe et al, 1994) and our resistant lines are the result of a selection for a broad mildew resistance to three different isolates during the development of a pre-breeding material from different sources of the entire rye genome. While chromosome 1R is regarded as the major contributor (Zeller, 1973), the involvement in the defence reaction and nature of resistance from the other rye chromosomes remains to be investigated.
Aberrant forms of chromosomes have not been reported extensively in wheat alien lines, although they are probably more commonly occurring than indicated by documented cases (Taketa et al, 1997). C-banding could identify the presence of a structural aberrant chromosome, while it was necessary to use detailed and comprehensive molecular cytogenetic methods to describe the aberrant chromosome as a pericentric inversion of rye chromosome 1R (Figure 3). GISH suggested that the chromosome is composed of rye chromatin (Figure 2g) while the use of five different repetitive DNA sequences with distinct distribution patterns on rye chromosomes (Cuadrado et al, 1995; Cuadrado and Schwarzacher, 1998) detected the inversion and localised the inversion breakpoints on the long and short arm (Figure 3). As the original 1R and the der1R shared the same number of hybridisation sites and distances between the sites, albeit their order being reversed in the middle of the chromosome, it is unlikely that material from other rye or wheat chromosomes is involved in the rearrangement.
The der1R in the lines 7–102 and 7–169 showed the same pattern of resistance as found in line 7–225, which had a normal 1R(1D) and derived from the same population, without any obvious effects on the resistance caused by the inversion. GISH excluded the presence of rye segments other than 1R in all the three substitution lines making 1R, normal or derivative, the sole source of the acquired mildew resistance. The lines with the der1R are more difficult to maintain in crosses with other lines carrying 1R and contain no value of increased resistance over the original wheat cultivar per se. Instead, they could be used for the development of recombinant wheat-rye lines. These recombinant lines are particularly desired by plant breeders to retain useful genes while minimising the number of deleterious characters from the alien species by reducing the size of the incorporated alien chromatin (Koebner et al, 1986). Several strategies have been developed to induce recombination such as using ionising radiation, through manipulation of the genetic control of homoeologous chromosome pairing or generating secondary recombinants by crosses between two lines sharing homologous regions of different length (see Koebner et al, 1986; Islam and Shepherd, 1991; Rogowsky et al, 1991). In combinations with normal or rearranged 1R, the two lines described here could be used to create duplications or deletions of certain defined chromosome regions. Merker and Forsström (2000) described another structural rearrangement of chromosome 1R and crosses between such lines could create a diversity of progeny with duplicated and deleted chromosome segments and specific genes of 1R transferred to the wheat genome.
Physical and genetical mapping, besides understanding the organisation and evolution of genomes (Heslop-Harrison, 2000), have substantially increased the potential to enhance the efficiency of plant breeding. Correlation of physical and genetic maps of markers and genes can be achieved by in situ hybridisation and identifies regions of the genome rich in genes and recombination to be targeted in breeding programmes (Schwarzacher, 1996). In the present study, we showed that FISH was efficient in describing aberrant chromosomes. Selection of plant material could therefore be improved by means of molecular cytogenetic methods and different traits more accurately traced through the breeding programmes for the benefit of targeting the goals more exactly in plant breeding.
References
Castilho, A, Heslop-Harrison, JS (1995). Physical mapping of 5S and 18S-25S rDNA and repetitive DNA sequences in Aegilops umbellulata. Genome, 38: 91–96.
Cuadrado, A, Ceoloni, C, Jouve, N (1995). Variation in highly repetitive DNA composition of heterochromatin in rye studied by fluorescence in situ hybridization. Genome, 38: 1061–1069.
Cuadrado, A, Schwarzacher, T (1998). The chromosomal organization of simple sequence repeats in wheat and rye genomes. Chromosoma, 107: 587–594.
Cuadrado, A, Schwarzacher, T, Jouve, N (2000). Identification of different chromatin classes in wheat using in situ hybridization with simple sequence repeat oligonucleotides. Theor Appl Genet, 101: 711–717.
Driscoll, CJ, Jensen, NF (1965). Release of a wheat-rye translocation stock involving leaf rust and powdery mildew resistances. Crop Sci, 5: 279–280.
Friebe, B, Heun, M, Tuleen, N, Zeller, FJ, Gill, BS (1994). Cytogenetically monitored transfer of powdery mildew resistance from rye into wheat. Crop Sci, 34: 621–625.
Forsström, PO, Merker, A (2001). Sources of wheat powdery mildew resistance from wheat-rye and wheat-Leymus hybrids. Hereditas, 134: 115–119.
Gerlach, WL, Bedbrock, JR (1979). Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucl Acids Res, 7: 1869–1885.
Gerlach, WL, Dyer, TA (1980). Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucl Acids Res, 8: 4851–4865.
Gill, BS, Friebe, B, Endo, TR (1991). Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome, 34: 830–839.
Heslop-Harrison, JS (2000). Comparative genome organization in plants: from sequence and markers to chromatin and chromosomes. Plant Cell, 12: 617–635.
Heslop-Harrison, JS, Leitch, AR, Schwarzacher, T, Anamthawat-Jónsson, K (1990). Detection and characterization of 1B/1R translocations in hexaploid wheat. Heredity, 65: 385–392.
Heun, M, Friebe, B, Bushuk, W (1990). Chromosomal location of the powdery mildew resistance gene of Amigo wheat. Phytopathology, 80: 1129–1133.
Islam, AKMR, Shepherd, KW (1991). Production of wheat-barley recombinant chromosomes through induced homoeologous pairing. 1. Isolation of recombinants involving barley arm 3HL and 6HL. Theor Appl Genet, 83: 489–494.
Koebner, RMD, Shepherd, KW, Appels, R (1986). Controlled introgression to wheat of genes from rye chromosome arm 1RS by induction of allosyndesis. 2. Characterisation of recombinants. Theor Appl Genet, 73: 209–217.
Leitch, IJ, Leitch, AR, Heslop-Harrison, JS (1991). Physical mapping of plant DNA sequences by simultaneous in situ hybridization of two differently labelled fluorescent probes. Genome, 34: 329–333.
Lind, V (1982). Analysis of the resistance of wheat-rye addition lines to powdery mildew of wheat (Erysiphe graminis f. sp. tritici). Tagungsber Akad Landwirtsch Wiss DDR, 198: 509–520.
McIntyre, CL, Pereira, S, Moran, LB, Appels, R (1990). New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome, 33: 635–640.
Merker, A, Forsström, PO (2000). Isolation of mildew resistant wheat-rye translocation lines from a double substitution line. Euphytica, 115: 167–172.
Mukai, Y, Nakahara, Y, Yamamota, M (1993). Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome, 36: 489–494.
Murata, M, Nakata, N, Yasumuro, Y (1992). Origin and molecular structure of a midget chromosome in a common wheat carrying rye cytoplasm. Chromosoma, 102: 27–31.
Pedersen, C, Langridge, P (1997). Identification of the entire chromosome complement of breadwheat by two-colour FISH. Genome, 40: 589–593.
Rabinovich, SV (1998). Importance of wheat-rye translocations for breeding modern cultivars of Triticum aestivum L. Euphytica, 100: 323–340.
Rayburn, AL, Gill, BS (1986). Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol Biol Rep, 4: 102–109.
Rogowsky, PM, Guidet, FLY, Langridge, P, Shepherd, KW, Koebner, RMD (1991). Isolation and characterization of wheat-rye recombinants involving chromosome arm 1DS of wheat. Theor Appl Genet, 82: 537–544.
Schwarzacher, T (1996). The physical organization of Triticeae chromosomes. In: Heslop-Harrison JS (ed) Unifying Plant Genomes, Symposia of the Society for Experimental Biology, Number 50, The Company of Biologists Ltd: Cambridge, pp 71–75.
Schwarzacher, T, Leitch, AR, Bennett, MD, Heslop-Harrison, JS (1989). In situ localization of parental genomes in a wide hybrid. Ann Bot, 64: 315–324.
Schwarzacher, T, Anamthawat-Jónsson, K, Harrison, GE, Islam, AKMR, Jia, JZ, King, IP, et al (1992). Genomic in situ hybridization to identify alien chromosomes and chromosome segments in wheat. Theor Appl Genet, 84: 778–786.
Schwarzacher, T, Heslop-Harrison, JS (2000). Practical in situ hybridization. BIOS Scientific Publishers Ltd.
Sybenga, J (1983). Rye chromosome nomenclature and homoeology relationships, workshop report. Z Pflanzenzucht, 90: 297–304.
Taketa, S, Nakazaki, T, Schwarzacher, T, Heslop-Harrison, JS (1997). Detection of a 4DL chromosome segment translocated to rye chromosome 5R in an advanced hexaploid triticale line Bronco 90. Euphytica, 97: 91–96.
Vershinin, A, Svitashev, S, Gummeson, P-O, Salomon, B, Bothmer, R Von, Bryngelsson, T (1994). Characterization of a family of tandemly repeated DNA sequences in Triticeae. Theor Appl Genet, 89: 217–225.
Vershinin, AV, Schwarzacher, T, Heslop-Harrison, JS (1995). The large-scale genomic organization of repetitive DNA families at the telomeres of rye chromosomes. The Plant Cell, 7: 1823–1833.
Zeller, FJ (1973). 1B/1R wheat-rye chromosome substitutions and translocations. In: Proceedings of the 4th International Wheat Genetics Symposium. pp 209–222.
Acknowledgements
This research was supported by a travel grant from the Royal Swedish Academy of Agriculture and Forestry, and carried out at the John Innes Centre, Norwich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsström, PO., Merker, A. & Schwarzacher, T. Characterisation of mildew resistant wheat-rye substitution lines and identification of an inverted chromosome by fluorescent in situ hybridisation. Heredity 88, 349–355 (2002). https://doi.org/10.1038/sj.hdy.6800051
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.hdy.6800051
Keywords
This article is cited by
-
Introgression of chromosome segments from multiple alien species in wheat breeding lines with wheat streak mosaic virus resistance
Heredity (2016)
-
An assessment of karyotype restructuring in the neoallotetraploid Tragopogon miscellus (Asteraceae)
Chromosome Research (2013)
-
Agropyron elongatum chromatin localization on the wheat chromosomes in an introgression line
Planta (2005)