Abstract
Purpose
To evaluate intraocular lens (IOL) power calculation using ray tracing in patients presenting with cataract after excimer laser surgery.
Methods
Ten eyes of seven consecutive patients who presented for cataract surgery following excimer laser treatment without any pre-refractive biometry data were enrolled in this prospective clinical study. Preoperatively, IOL power calculation was performed using a ray tracing software called OKULIX. Keratometry data (C-Scan) were imported and axial length (IOLMaster) was entered manually. Accuracy of IOL power calculation was investigated by subtracting attempted and achieved spherical equivalent.
Results
Mean spherical equivalent was −3.51±2.77 D (range −10.38 to −0.5 D) preoperatively and −1.01±1.08 D (range −2.5 to +0.75 D) postoperatively. Mean error was 0.31±0.84 D, mean absolute error was 0.74±0.46 D, and IOL calculation errors ranged from −1.39 to +1.47 D. A total of 40% of eyes were within ±0.5 D, 70% within ±1.0 D, and 100% within ±1.5 D. Three eyes with corneal radii over 10 mm showed calculation errors exceeding ±1.0 D. Mean best-corrected visual acuity increased from 20/60 to 20/30 postoperatively.
Conclusions
IOL power calculation after excimer laser surgery can be difficult, especially when pre-refractive keratometry values are not available. In these cases, ray tracing combined with corneal topography measurements provides reliable and satisfactory postoperative results. However, it is advisable to be careful when calculating IOL power for eyes with corneal radii exceeding 10 mm because of slightly higher prediction errors.
Similar content being viewed by others
Introduction
The number of patients presenting with cataract formation after refractive surgery is increasing over time. Accurate intraocular lens (IOL) power calculation is even more important for these patients, as they are used to being spectacles-independent and have high demands regarding the postoperative refractive outcome. However, determination of accurate keratometry (K)-readings after refractive surgery is difficult, as corneal shape was changed by the refractive treatment. Standard keratometers measure the refractive corneal power in a paracentral region, which is assumed to be slightly prolate or spherical, respectively. Owing to the change from sphere to asphere after refractive surgery, measurements of corneal radii and power, respectively, are very likely to be inaccurate.1, 2 Corneal power is overestimated followed by an underestimation of IOL power resulting in hyperopia postoperatively.3, 4 Moreover, this overestimation of K-readings is increased by a reduced refractive corneal index, which is determined by the two refractive surfaces of the cornea. Removing tissue using an excimer laser alters the relationship between anterior and posterior surface.5
In order to circumvent this problem of inaccurate keratometry measurements, it would be helpful to know the original, pre-refractive biometric data. However, in many cases, these values are not available. During the last 15 years, different methods were presented to solve this problem, which either depend on pre-refractive data or do not need these values.6, 7, 8, 9, 10, 11
Recently, a new software using numerical ray tracing is available called OKULIX (Ingenieurbüro der Leu, Hillerse, Germany). The program was developed at the University of Mainz, Germany, and has been explained in detail.11, 12, 13 It is able to determine the monochromatic optical capacities of the pseudophakic, human eye. Single light rays limited only by pupillary size are evaluated. This is a main difference between OKULIX and standard IOL calculation formulas based on Gaussian optics, which are valid only for paraxial rays.11 Ray tracing calculation is made from fovea to cornea, which is allowed because geometric optical pathway can always be mirrored.11 Rays undergo different refractions on different surfaces, where the refractive index changes (intravitreal, lens, aqueous humour, cornea). The shape of the surfaces is mainly described by their central curvature radii.11 Additionally, IOL data, for example radius, thickness, and refractive index, as well as corneal topography measurements are included in the IOL calculation. Finally, the retinal image quality of blurred Landolt rings can be displayed.
The purpose of this study was to investigate the accuracy of the IOL calculation using OKULIX following excimer laser surgery by comparing attempted vs achieved postoperative refractive outcome.
Patients and methods
We evaluated the OKULIX software in a prospective, noncomparative interventional case series performed at the Department of Ophthalmology, University of Heidelberg, Germany.
We calculated the IOL power for 10 eyes of seven cataract patients using OKULIX. Each eye underwent refractive laser treatment for myopia between 1985 and 2003 (photorefractive keratectomy (PRK): n=6; laser in situ keratomileusis (LASIK): n=4). Mean patient age was 58.9±4.7 years (range 53–65 years).
Different IOL calculation steps were completed: A corneal topography measurement was performed using the C-Scan (Technomed GmbH, Baesweiler, Germany). Axial length was evaluated using the IOLMaster (Carl Zeiss Meditec AG, Jena, Germany). In the next step, we exported the results of the C-Scan examination and imported them into the OKULIX software. Afterwards, the user had to determine the eye and the IOL type from a given catalogue. Axial length as well as target refraction was entered, and corneal radii were shown automatically (Figure 1). It was now important to change the acoustical value for axial length to the original, optical value. After determination of the biometry system, the postoperative anterior chamber depth (ACD) was proposed. Finally, the IOL power was calculated and several neighbouring lenses were shown together with their postoperative refractive error (Figure 2).
In the cases presented herein, uneventful phacoemulsification in topical or general anaesthesia was performed using clear cornea or sclero-corneal incision technique. AR40e IOLs (AMO Germany GmbH, Ettlingen, Germany) were implanted with IOL powers ranging between 12 and 22 D (mean IOL power: 18.15±2.73 D) using Sapphire or Emerald injectors (AMO Germany GmbH, Ettlingen, Germany).
Accuracy of IOL power calculation using OKULIX
Postoperatively, the accuracy of IOL power calculation was evaluated by comparing attempted vs achieved spherical equivalent. Postoperative refraction was obtained at least 3 months after surgery. Mean prediction error as well as absolute error was determined.
Contribution of C-Scan central corneal power measurements to IOL calculation
In order to assess the relative contribution of the C-Scan keratometry readings to IOL calculation using standard technology, we entered these data into the IOLMaster and calculated IOL power using Haigis and Holladay formulas. Finally, these results were compared to the OKULIX outcome in terms of mean prediction and absolute errors. Additionally, we compared K-readings measured with IOLMaster and C-Scan-derived corneal power values used for the OKULIX calculation process.
Statistically analysis was performed using the Wilcoxon test and a P-value less than 0.05 was considered statistically significant.
Results
Accuracy of IOL power calculation using OKULIX
Mean spherical equivalent of all patients was −3.51±2.77 D (range −10.38 to −0.5 D) preoperatively and −1.01±1.08 D (range −2.5 to +0.75 D) postoperatively. Average target refraction was −1.32±0.96 D (range −2.6 to +0.05 D). Comparing attempted vs achieved spherical equivalent, a mean error of 0.31±0.84 D was calculated (Figure 3). Mean absolute error was 0.74±0.46 D and IOL calculation errors ranged from −1.39 to +1.47 D. A total of 40% of eyes were within ±0.5 D, 70% within ±1.0 D, and 100% within ±1.5 D. Table 1 shows single values for each evaluated eye regarding preoperative, target, and postoperative refraction as well as prediction and absolute error. Six of 10 eyes (60%) showed a hyperopic outcome, which should be taken into account when choosing an IOL power. Three eyes with corneal radii over 10 mm showed calculation errors exceeding ±1.0 D (Table 1, eyes number 5, 8, and 9), indicating a tendency for higher prediction errors under these conditions. Mean best-corrected visual acuity increased from 20/60 to 20/30 postoperatively. Five eyes (50%) did not achieve 20/25 or better because of former retinal detachment or eccentric corneal ablation zones.
Contribution of C-Scan central corneal power measurements to IOL calculation
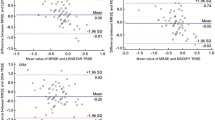
Mean prediction error using C-Scan keratometry data in combination with standard technology (IOLMaster) was 1.34±1.30 D (median 1.36 D, Haigis formula) and 2.19±1.70 D (median 2.07 D, Holladay formula), respectively. As far as absolute errors are concerned, the following mean values were calculated: 1.60±0.93 D (median 1.41 D, Haigis formula) and 2.42±1.31 D (median 2.07 D, Holladay formula). Nine of 10 eyes (90%) would have had a hyperopic refractive outcome. The difference between OKULIX and standard technology used for IOL calculation was statistically significant (Wilcoxon test, P<0.05) (Figure 4).
Mean K1-readings were 9.08±1.06 mm (Zeiss IOLMaster, range 7.46–10.92 mm) and 9.38±1.28 mm (C-Scan, range 7.48–11.63 mm). Mean K2-values calculated were as follows: 8.80±0.95 mm (Zeiss IOLMaster, range 7.38–10.61 mm) and 8.99±1.05 mm (C-Scan, range 7.44–11.1 mm). A statistically significant difference was calculated between the two systems in terms of K1 and K2 measurements (Wilcoxon test, P<0.01). Using IOLMaster K-readings for the IOL calculation would have led to an even greater overestimation of corneal refractive power, increasing the risk for postoperative hyperopia significantly.
Discussion
At the moment, different methods are used in order to solve the problem of inaccurate IOL calculation after refractive surgery. They can be divided into two groups: on the one hand, there are methods that require the original, pre-refractive data, like clinical history,6, 7 Feiz–Mannis,14 double-K,15 or adjusted effective refractive power (EffRPadj) method.5, 16 On the other hand, IOL power calculation is possible without the original data using contact lens over-refraction method,6, 7 correlating axial length and corneal radius correcting factors,17 corneal topography (Orbscan II),18 or ray tracing.11, 12, 13
The clinical history method (CHM) is considered to be the most reliable procedure to estimate corneal power values after refractive surgery.3, 4, 19, 20, 21, 22, 23 The spherical equivalent change is subtracted from the original corneal refractive power. However, accurate pre-refractive data as well as a stable postoperative refraction are necessary.6, 7, 8 Several reports have been published indicating the high accuracy of this method.3, 4, 19, 20, 21, 22, 23 Odenthal et al19 compared eight possible methods of assessing K-readings in combination with three different IOL calculation formulas (Hoffer Q, Holladay 1, SRK/T) after photorefractive keratectomy. The CHM corrected by the spectacle plane change in refraction in combination with the Hoffer Q formula provided the most accurate results. A total of 70% of eyes showed a predicted refraction within 1 D of achieved postcataract refraction. Latkany et al20 compared six methods to an exact IOL that would have resulted in emmetropia. The clinical history at the spectacle plane provided the best postoperative outcome with a mean deviation from emmetropia of −0.56±1.59 D. Argento et al21 evaluated five methods and three calculation formulas and reported best accuracy using CHM in combination with Hoffer Q (absolute error: 1.11±0.67 D) and Holladay 2 (absolute error: 1.45±1.16 D). Findings of Ladas et al,22 Gimbel et al,4 and Randleman et al,23 also support the good outcomes for CHM.
Wang et al24 compared various combinations of different calculation methods and four IOL formulas. The most accurate method was the combination of a double-K formula and corneal values derived from EffRPadj. The Feiz–Mannis method was comparable with double-K and EffRPadj. However, it showed the largest variance indicating a poorer consistency. No significant difference was found between the formulas, indicating that the method of calculation is more important to find the appropriate corneal power value.
Unfortunately, the pre-refractive keratometry data are often not available, which makes the IOL calculation even more difficult. The hard contact lens method (CLM)6, 7, 25 has been considered to be the method of choice when original values are missing. It determines the difference between the manifest postoperative refraction with and without a plano hard contact lens of known base curve and subtracts this difference from the base curve. However, visual acuity must be good enough and a changed refraction due to lens opacity can lead to inaccurate results. Moreover, Haigis25 explained the possibility of K-readings overestimation of approximately 1.0–1.5 D in eyes that have had refractive surgery for myopia of −5.0 to −10.0 D.25 His findings are in accordance with other studies, which revealed that the contact lens over-refraction method was not reliable.21, 24 According to Argento et al,21 corneal topography seems to be more accurate than CLM and conventional keratometry and thus should be the method of choice when pre-refractive K-readings are not available. Its advantage is that more points spread over a larger corneal area are measured compared to conventional keratometry. Sonego-Krone et al,18 showed that Orbscan II total-mean and total-optical power maps accurately assess the corneal power after myopic LASIK. However, there are reports indicating that refractive outcome using corneal topography has not been satisfying.3, 22 Unfortunately, corneal topography and keratometry systems calculate the refractive corneal power using a standardized refractive index (eg 1.3375) to convert corneal radii into dioptres. As the relationship between anterior and posterior surface of the cornea is changed by the excimer laser treatment, topography or keratomety values should be modified.5
When reviewing the literature, it becomes obvious that each method has its advantages and its disadvantages. When the pre-refractive data are available, the postoperative outcome seems to be more predictable using clinical history, double-K, or EffRP method. Without these original data, IOL calculation remains even more difficult. It is important and advisable to compare several calculation methods and to use the lowest corneal refractive power in dioptres. In general, patients should be informed that there is a potential risk for inaccurate IOL power calculation after refractive surgery. A corneal topography measurement can be helpful to detect asphericity as well as irregular astigmatism. In combination with numerical ray tracing (OKULIX), IOL calculation is reliable after excimer laser surgery, especially when the pre-refractive keratometry data are not available. However, at the moment, only specific corneal topography and biometry systems can be used in combination with the program, for example, Technomed C-Scan, Oculus Keratograph, Tomey 2/4, Zeiss/Humphrey Atlas, Zeiss IOLMaster, Tomey Biometer. Moreover, accuracy of biometry measurements, errors in the IOL data given by the manufacturer as well as postoperative anterior chamber depth prediction remain a problem.11, 12, 13, 26
Our evaluation of the OKULIX software showed good results. In 70% of the eyes, the difference between attempted vs achieved spherical equivalent was within ±1.0 D with a mean absolute error of 0.74±0.46 D. However, six of 10 eyes (60%) showed a hyperopic outcome, which needs to be taken into account when choosing an IOL power. There was a tendency for slightly higher prediction errors exceeding 1 D in cases with corneal radii over 10 mm. However, standard technology for IOL calculation (IOLMaster) even in combination with C-Scan-derived keratometry data would have led to more hyperopic refractive outcomes (90%).
For better comparison of different calculation methods, refractive surgeons should provide information on pre- and postoperative refraction as well as preoperative K-readings to their patients in order to achieve a satisfactory refractive outcome after cataract surgery and prevent hyperopic surprises.
References
Hamilton DR, Hardten DR . Cataract surgery in patients with prior refractive surgery. Curr Opin Ophthalmol 2003; 14: 44–53.
Koch DD, Liu JF, Hyde LL, Rock RL, Emery JM . Refractive complications of cataract surgery after radial keratotomy. Am J Ophthalmol 1989; 108: 676–682.
Seitz B, Langenbucher A, Nguyen NX, Kus MM, Kuchle M . Underestimation of intraocular lens power for cataract surgery after myopic photorefractive keratectomy. Ophthalmology 1999; 106: 693–702.
Gimbel HV, Sun R, Furlong MT, van Westenbrugge JA, Kassab J . Accuracy and predictability of intraocular lens power calculation after photorefractive keratectomy. J Cataract Refract Surg 2000; 26: 1147–1151.
Hamed AM, Wang L, Misra M, Koch DD . A comparative analysis of five methods of determining corneal refractive power in eyes that have undergone myopic laser in situ keratomileusis. Ophthalmology 2002; 109: 651–658.
Holladay JT . Consultations in refractive surgery. Refract Corneal Surg 1989; 5: 203.
Hoffer KJ . Intraocular lens power calculation for eyes after refractive keratotomy. J Refract Surg 1995; 11: 490–493.
Hoffer KJ . Calculating intraocular lens power after refractive corneal surgery. Arch Ophthalmol 2002; 120: 500–501.
Seitz B, Langenbucher A, Haigis W . [Pitfalls of IOL power prediction after photorefractive keratectomy for high myopia—case report, practical recommendations and literature review]. Klin Monatsbl Augenh 2002; 219: 840–850.
Langenbucher A, Haigis W, Seitz B . Difficult lens power calculations. Curr Opin Ophthalmol 2004; 15: 1–9.
Preussner PR, Wahl J, Lahdo H, Dick B, Findl O . Ray tracing for intraocular lens calculation. J Cataract Refract Surg 2002; 28: 1412–1419.
Preussner PR, Wahl J . [Consistent numerical calculation of optics of the pseudophakic eye]. Ophthalmologe 2000; 97: 126–141.
Preussner PR, Wahl J, Lahdo H, Findl O . [Consistent IOL-calculation]. Ophthalmologe 2001; 98: 300–304.
Feiz V, Mannis MJ, Garcia-Ferrer F, Kandavel G, Darlington JK, Kim E et al. Intraocular lens power calculation after laser in situ keratomileusis for myopia and hyperopia. Cornea 2001; 20: 792–797.
Aramberri J . Intraocular lens power calculation after corneal refractive surgery: double-K method. J Cataract Refract Surg 2003; 29: 2063–2068.
Holladay JT . Corneal topography using the Holladay Diagnostic Summary. J Cataract Refract Surg 1997; 23: 209–221.
Rosa N, Capasso L, Romano A . A new method of calculating intraocular lens power after photorefractive keratectomy. J Refract Surg 2002; 18: 720–724.
Sonego-Krone S, Lopez-Moreno G, Beaujon-Balbi OV, Arce CG, Schor P, Campos M . A direct method to measure the power of the central cornea after myopic laser in situ keratomileusis. Arch Ophthalmol 2004; 122: 159–166.
Odenthal MT, Eggink CA, Melles G, Pameyer JH, Geerards AJ, Beekhuis WH . Clinical and theoretical results of intraocular lens power calculation for cataract surgery after photorefractive keratectomy for myopia. Arch Ophthalmol 2002; 120: 431–438.
Latkany RA, Chokshi AR, Speaker MG, Abramson J, Soloway BD, Yu G . Intraocular lens calculations after refractive surgery. J Cataract Refract Surg 2005; 31: 562–570.
Argento C, Cosentino MJ, Badoza D . Intraocular lens power calculations after refractive surgery. J Cataract Refract Surg 2003; 29: 1346–1351.
Ladas JG, Boxer Wachler BS, Hunkeler JD, Durrie DS . Intraocular lens power calculations using corneal topography after photorefractive keratectomy. Am J Ophthalmol 2001; 132: 254–255.
Randleman JB, Loupe DN, Song CD, Waring 3rd GO, Stulting RD . Intraocular lens power calculations after laser in situ keratomileusis. Cornea 2002; 21: 751–755.
Wang L, Booth MA, Koch DD . Comparison of intraocular lens power calculation methods in eyes that have undergone LASIK. Ophthalmology 2004; 111: 1825–1831.
Haigis W . Corneal power after refractive surgery for myopia: contact lens method. J Cataract Refract Surg 2003; 29: 1397–1411.
Preussner PR, Wahl J, Weitzel D, Berthold S, Kriechbaum K, Findl O . Predicting postoperative intraocular lens position and refraction. J Cataract Refract Surg 2004; 30: 2077–2083.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no financial or proprietary interest in any of the products mentioned
Rights and permissions
About this article
Cite this article
Rabsilber, T., Reuland, A., Holzer, M. et al. Intraocular lens power calculation using ray tracing following excimer laser surgery. Eye 21, 697–701 (2007). https://doi.org/10.1038/sj.eye.6702300
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702300
Keywords
This article is cited by
-
Intraocular Lens Power Calculation after Small Incision Lenticule Extraction
Scientific Reports (2020)
-
Accuracy of minus power intraocular lens calculation using OKULIX ray tracing software
International Ophthalmology (2019)
-
Intraocular lens power calculation in eyes with previous corneal refractive surgery
Eye and Vision (2018)
-
Improving accuracy of corneal power measurement with partial coherence interferometry after corneal refractive surgery using a multivariate polynomial approach
BioMedical Engineering OnLine (2018)
-
Intraocular lens power calculation following laser refractive surgery
Eye and Vision (2015)