Abstract
Purpose
In a nonrandomized, prospective study the efficacy of radiotherapy with 16 and 20 Gray (Gy) for subfoveal neovascularization in age-related macular degeneration (ARMD) was analysed.
Material and Methods
From 1996 to 1998, 63 eyes were irradiated with 16 Gy and 38 eyes with 20 Gy for exudative ARMD. A total of 12 eyes had classic ARMD, 89 eyes occult ARMD, median baseline visual acuity (VA) was 6/30 (range: 3/60–6/9.5), median age was 78 years. Risk factors (type of ARMD, baseline VA) were evenly distributed in both groups. Median follow-up was 1.3 years (range: 4 months–4.7 years). VA of ±1 line or better and unchanged size and activity of the membrane in fluorescein angiography were defined as stable. Actuarial methods were used.
Results
Median loss of VA was −3 lines (range: −14 to +5), neovascularization remained unchanged or decreased in size and activity in 35 eyes. At 18 months, the probability of stabilized VA was 0.4 (95% confidence interval (CI): 0.3–0.5), at 24 months 0.3 (95% CI: 0.2–0.4). Radiation dose, type of ARMD or baseline VA had no significant impact on outcome of VA and membrane size and activity (P>0.05). Side effects were mild and transient increased tearing.
Conclusion
In this study, the results after radiotherapy were comparable to the natural course of the disease. An impact of radiation dose (16 vs20 Gy) on stabilizing visual acuity and subfoveal neovascularization could not be shown. The results of studies on dose escalation using very small fields and high radiation doses should be awaited.
Similar content being viewed by others
Introduction
The exudative form of age-related macular degeneration (ARMD) accounts for the majority of the patients with ARMD registered as blind.1 It is characterized by the formation of a subfoveal choroidal neovascularization (CNV), frequently associated with exudation of fluid, haemorrhage, and retinal detachment. Most of the lesions are not amenable to laser therapy because of their vicinity to the fovea. Earlier studies suggested that radiotherapy may inhibit further loss of visual acuity but following studies rendered contradictory results.2, 3, 4, 5, 6 A randomized double-blinded study failed to demonstrate any benefit for patients treated with 16 Gy. However, in a randomized study, patients treated with high radiation doses (24 Gy, 6 Gy per fraction) had significantly better visual acuity than the untreated controls.7 The dose–response relation of radiation of CNV remains to be established before abandoning radiotherapy for ARMD.8 This is a prospective study of two different radiation doses.
Materials and methods
In a prospective, nonrandomized study, patients with the classic or occult type of ARMD who did not meet the criteria of small, well-defined subfoveal choroidal neovascularization (CNV), as defined by the Macular Photocoagulation Study Group (MPSG),9 were treated with radiotherapy. To be eligible, patients had to have clinical signs of exudative ARMD, a visual acuity (VA) of ⩽6/9.5 and ⩾3/60, and no signs of retinopathy. Patients with classic CNV were eligible because photodynamic therapy with verteporfin was not proven or available at the time of study. Eyes with conditions relevant to visual acuity other than the membrane or exudation itself (mostly haemorrhage near the macula at baseline examination or planned lens surgery during follow-up) were excluded from the study.
The baseline evaluation included binocular indirect ophthalmoscopy, slit-lamp biomicroscopy, the testing of VA, and fluorescein angiography (FAG). VA was determined monocular with best correction, at 5 m distance with figures of uniform luminescence. For FAG, the pupil was dilated and the first picture was taken in red-free light. After injection of 5 ml fluorescein (5% concentration), pictures were taken in quick succession every second. Late photographs were taken after 5–10 min.
Radiotherapy was initiated after obtaining the patient's informed consent. The head was immobilized with a thermoplastic head mask in the supine position. Irradiation was administered with a lateral treatment portal angled posteriorly 5° and shaped with a D-absorber to spare the ipsi- and contralateral lens, as well as surrounding structures. The field position and dose distribution were checked with a CT-assisted three-dimensional planning system and, if necessary, corrected. Patients were treated with 6 MeV photons from a linear accelerator at 1 m source skin distance with fractions of 2 Gy 5 days per week. From December 1996 to February 1998, a total dose of 16 Gy was administered in the macular region; from March 1998 onwards, the total dose was raised to 20 Gy. Follow-up visits were scheduled 3, 12, and 24 months after treatment. The follow-up examinations included ophthalmoscopy, slit-lamp biomicroscopy, testing of VA and FAG, as well as evaluation of possible radiation sequelae by a radiation-oncologist.
Patients' characteristics
From December 1996 to December 1998, 169 eyes were treated. In all, 31 eyes did not meet the inclusion criteria because of conditions relevant to VA other than the membrane or exudation itself (mostly haemorrhage in the macular region at baseline examination or planned lens surgery during follow-up). A total of 31 patients/eyes were lost to follow up immediately after therapy. In most cases, the elderly patients were not able or unwilling to travel large distances to the clinic. Six patients refused FAGs. In all, 14 of 37 eyes lost to follow up had been treated with 16 Gy and 23 of 37 eyes with 20 Gy.
The study group consisted of 88 patients/101 eyes who were available for follow-up. The median age was 78 years (range: 52–98). In all, 12 eyes had classic and 89 eyes occult ARMD (Table 1). Median baseline VA was 6/30 (range: 3/60–6/9.5). A total of 63 eyes (62%) were treated with 16 Gy and 38 eyes (38%) with 20 Gy total reference dose. The type of ARMD, baseline VA and time of follow-up were evenly distributed between dose groups. All patients received the planned radiation dose. Radiation sequela was mild and transient increased tearing. The median follow-up time was 1.3 years (range: 4 months–4.7 years). A total of 95 eyes had a minimum follow-up of 10 months and 38 eyes at least 2 years follow-up.
Evaluation
The primary end point was the rate of stabilization of VA 18 months after radiotherapy. VA of ±1 line or better to the base line evaluation was defined as stable.
Considering the time course of the disease, follow-up is usually performed at approximately 1 year after therapy. In order to account for differing time of follow-up, statistical analysis was performed with the Kaplan–Meier method. As secondary end point, the actuarial rate of severe loss of vision, defined as loss of ⩾5 lines, was estimated. To facilitate comparison with previous studies, the crude rate of stabilization of VA 10–18 months after radiotherapy are stated.
FAGs were evaluated with respect to the criteria of activity and size of the subfoveolar lesion by two experienced ophthalmologists blinded to the total dose. Enlargement of size and/or activity was defined as progressive disease. The actuarial rate of stable SNV, that is, unchanged or decreased size and activity, was estimated.
The impact of prognostic and treatment factors on VA and morphology of the disease were analysed with the log-rank test and Cox proportional hazards estimation.
Results
During the entire follow-up, the VA changed by a median of −3 lines (range: −14 to +5) and severe loss of vision (⩾5 lines) occurred in 40 eyes (40%), vision improved or remained stable in 35 eyes (35%) (Table 2). At 2 years, 11 of 38 eyes had stable or improved VA, eight of 23 eyes in the 16 Gy group and three of 15 in the 20 Gy group.
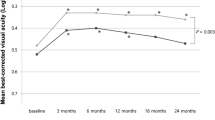
At 12 months after treatment, the probability of stabilized VA was 0.6 (95% confidence interval (CI): 0.5–0.8) overall, 0.6 (95% CI: 0.5–0.7) in the 16 Gy group and the 20 Gy group alike, P=0.9 (Figure 1). At 18 months, the probability was 0.4 (95% CI: 0.3–0.5) overall, 0.4 (95% CI: 0.2–0.5) in the 16 Gy group and 0.4 (95% CI: 0.3–0.6) in the 20 Gy group. It remained nearly unchanged until 24 months after treatment. At 18 months, the probability of less than five lines loss of VA was 0.6 (95% CI: 0.5–0.7) in the 16 Gy group and 0.8 (95% CI: 0.7–0.9) in the 20 Gy group; the difference not significant (P=0.7).
Impact of treatment, 16 vs 20 Gy total dose, on VA. Stable VA is defined as ±1 line or better than baseline examination. The probability of stable VA is estimated with Kaplan–Meier analysis. Patients at risk (in follow-up) overall: 16 Gy, n=63; 20 Gy, n=38. At 12 months: 16 Gy, n=34 (57); 20 Gy, n=23 (38). At 24 months: 16 Gy, n=9 (23); 20 Gy, n=9 (15).
At 18 months, the probability of unchanged size and/or activity of the subfoveal lesions was 0.5 (95% CI: 0.3–0.6) for the 16 Gy group as well as the 20 Gy group (P=0.7). Visual loss and morphologic changes correlated (P=0.01); however, FAG findings were a weak predictor of visual loss.
In multivariate analysis, the impact of the form of ARMD (occult vs classic), low baseline VA as indicator of advanced disease (⩽6/30 vs >6/30) and total dose (16 vs 20 Gy) had no significant impact either on the probability of retaining stable vision (P>0.05) or the probability of unchanged or decreased size/activity of subfoveolar neovascularization. The impact of initial lesion size was not studied.
Discussion
The prognosis of subfoveal CNV is poor. VA will deteriorate to 6/60 within 18 months in approximately 70% of the affected eyes.10 The pathogenesis of ARMD is complex. Degenerative changes in the retinal layers result in lipofuscein-related metabolism changes and tissue hypoxia that upregulate growth factors, especially vascular epithelial growth factor (VEGF), in the retinal pigment epithelium. Growth factors stimulate endothelial proliferation and new vessel formation and may be responsible for vessel permeability change and leakage. Capillary proliferation may also be induced by peroxidated lipids and other fatty-acid products (prostaglandin precursors).11, 12, 13, 14 In surgically removed CNV, an inflammatory reaction including macrophages and foreign body giant cells has been observed that may stimulate CNV growth.15, 16
Radiotherapy was suggested as a promising, noninvasive treatment option. In vitro and in vivo experiments demonstrated that irradiation inhibits the proliferation of endothelial cells, reduces the neovascular component of healing ocular wounds and may induce vessel obliteration.17, 18, 19, 20 Low-dose radiation (⩽25 Gy) has been shown to cause DNA-breaks, decrease cell replication and reduce prostacyclin synthesis and prostacyclin production.21, 22, 23 Radiation also increases cell permeability and induces vascular cell apoptosis in vitro.24, 25 The rationale for radiotherapy of ARMD with fairly low doses is to suppress the inflammatory and exudative component of ARMD as well as to inhibit further pathologic endothelial cell proliferation.17, 18, 26 All of these features were demonstrated in experimental choroidal neovascularization in rabbit eyes.27 The degenerative process itself and the persistence of the stimuli that upregulate the growth factors are probably not altered by radiotherapy.28
A variety of dose schedules has been employed in the recent decade. Low-dose radiotherapy with 4 × 0.2–0.5 Gy was ineffective.29
More often, a higher but safe dose of 10–16 Gy with 2 Gy per fraction was chosen. In an earlier study, external beam radiation therapy with 10–15 Gy (fraction size 2–3 Gy) appeared to be effective.4 The mean change in VA of treated eyes was less than 1 Bailey–Lovie line as opposed to a mean loss of 3.7 and 4.5 lines in untreated eyes at 1 and 2 years, respectively. However, the results of further prospective studies were disappointing.6, 28, 30, 31, 32 In a group of 91 patients, radiotherapy with 10 Gy in five fractions was not effective in comparison to a historical, well-defined, control group of untreated patients.28 In an Austrian study with 14.4 Gy in eight fractions,30 a German study of 73 patients with classic ARMD with 16 Gy in eight fractions,31 and a similar study of 69 patients with classic or occult CNV, the VA at 1 year was similar to the expected natural course of disease (25, 37, and 38%, respectively).6 Finally, a German multicentre study, testing 16 Gy in eight fractions vs sham irradiation in 205 patients, has proven radiotherapy with this dose to be ineffective.32 It is noteworthy that the double-masked design of the study avoids any uncertainties caused by the testing of VA, which is subjective. Also, a dose of 14 Gy in seven fractions vs sham irradiation seemed to show no benefit in a double-masked study of 82 patients and was closed early.33
The results of our study are within the range of similar studies. Within 18 months follow-up, the mean loss of VA was −3 lines and the actuarial rate of stable VA was 06/15. This is comparable to the expected natural course.10 There was a clear drop of VA 12–18 months after radiotherapy as has been shown by Schittkowski et al.34
Considering a possible dose relationship,5, 21, 25, 35 in this institution, the dose was raised to 20 Gy in 10 fractions in 1998. Only mild acute radiation effects (transient tearing) were observed. However, the higher dose did not improve the results in this study group. However, although prognostic relevant factors were evenly distributed between groups, this was a nonrandomized study and the sample size was only sufficient to detect a benefit of minimum 27% (α error 0.05, power 80%).
Stalmans et al36 reported that irradiation with 20 Gy in 10 fractions failed to control the growth of CNV and was ineffective in stabilizing vision in a group of 89 patients with initial low VA. In a small, randomized nonblinded study with 20 Gy in 10 fractions vs nil (including patients with minimum VA of 6/600), the average loss of VA at 6 months did not differ between the groups.37 Comparison of the studies is limited by differing inclusion criteria (predominant type of ARMD, initial vision) and definitions of loss of VA.
Considering the low mitotic activity of endothelial cells, high dose per fraction and high total doses may prove more effective than the conventional therapy with 10–20 Gy. To our knowledge, only two prospective dose escalation studies have been published and one study using 5 × 4 Gy is ongoing.9, 38, 39 Recently, a double-masked dose-escalation study on 1 Gy (4 × 0.25 Gy, control group), 8 Gy (4 × 2 Gy) and 16 Gy (4 × 4 Gy) in 150 patients was reported.38 Patients treated with 8 or 16 Gy lost significantly less VA than the 1 Gy group, but did not benefit of the higher dose (16 vs 8 Gy). Reading ability and size of the CNV worsened alike in all three groups. The rate of patients with stable VA was not stated. In a dose-escalation study with 14 Cobalt Gray Equivalent single-dose proton therapy, very good results with stabilization of VA in 90% of patients at 21 months were achieved.39
Studies using high-dose levels reported favourable results. In a randomized study of 74 patients, the patients treated with 4 × 6 Gy had significantly better preservation of vision than the untreated control group (50 vs 30%).8 A French group reported an impressive improvement of VA in 31% and stabilization in 33% of patients 18 months after treatment with 16–20 Gy in four to five fractions in a prospective, nonrandomized study.5
The radiation tolerance of eyes with ARMD may be reduced due to the underlying retinal changes so that the therapeutic window is probably small.5, 36, 40 So far, only one of the above studies using high dose per fraction has demonstrated an increased rate of radiation late effects: 15 of 212 patients developed retinopathy, choroidal teleangiectasia, optic neuropathy, or branch vein occlusion of varying severity within 2 years of treatment.5 This study reported the longest follow-up and had therefore a higher probability of recording late effects. It may be crucial to reduce the field size as far as possible if high doses per fraction are given. The small radiation volume in other high-dose studies may contribute to the lack of severe late effects.8, 39
Conclusion
In this study, the results after radiotherapy were comparable to the natural course of the disease. An impact of radiation dose (16 vs 20 Gy) on stabilizing VA and controlling the growth of the subfoveal choroidal membrane could not be shown. The results of studies on dose escalation using small radiation volumes should be awaited before abandoning radiotherapy in the treatment of ARMD.
References
Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA et al. The Framingham Eye Study monograph. Surv Ophthalmol 1980; 24(Suppl): 335–410.
Bergink GJ, Hoyng CB, van der Maazen RW, Vingerling JR, van Daal WA, Deutman AF . Visual acuity and scar size in eyes with age-related subfoveal choroidal neovascular lesions, 30 months after radiation therapy. Doc Ophthalmol 1996; 92: 61–75.
Chakravarthy U, Houston RF, Archer DB . Treatment of age-related subfoveal neovascular membranes by teletherapy: a pilot study. Br J Ophthalmol 1993; 77: 265–273.
Hart PM, Chakravarthy U, MacKenzie G, Archer DB, Houston RF . Teletherapy for subfoveal choroidal neovascularisation of age-related macular degeneratio: results of follow up in a non-randomised study. Br J Ophthalmol 1996; 80: 1046–1050.
Mauget-Faysse M, Chiquet C, Milea D, Romestaing P, Gèrard JP, Martin P et al. Long term results of radiotherapy for subfoveal choroidal neovascularistion in age related macular degeneration. Br J Ophthalmol 1999; 83: 923–928.
Gripp S, Stammen J, Petersen C, Hartmann A, Willers R, Althaus C . Radiotherapy in age-related macula degeneration. Int J Radiat Oncol Biol Phys 2002; 52: 489–495.
Bergink GJ, Hoyng CB, van der Maazen RWM, Vingerling JR, van Daal WA, Deutman AF . A randomized controlled clinical trial on the efficacy of radiation therapy in the control of subfoveal choroidal neovascularization in age-related macular degeneration: radiation versus observation. Graefe's Arch Clin Exp Ophthlamol 1998; 236: 321–325.
Fine SL, Maguire MG . It is not time to abandon radiotherapy for neovascular age-related macular degeneration. Arch Ophthalmol 2001; 119: 275–276.
Bressler NM, Frost LA, Bressler SB, Murphy RP, Fine SL . Natural course of poorly defined choroidal neovascularization associated with macular degeneration. Arch Ophthalmol 1988; 106: 1537–1542.
Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal lesions in age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol 1993; 111: 1200–1209.
Frank RN, Amin RH, Elliott D, Puklin JE, Abrams GW . Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol 1996; 122: 393–403.
Holz FG, Pauleikhoff D (eds). Altersabhängige Makuladegeneration. Springer : Berlin, 1997.
Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT . Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997; 81: 154–162.
Pauleikhoff D, Harper CA, Marshall J, Bird AC . Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology 1990; 97: 171–178.
Killingsworth HC, Sarks JP, Sarks SH . Macrophages related to Bruch's membrane in age-related macular degeneration. Eye 1990; 4: 613–621.
Lopez PF, Grossniklaus HE, Lambert MH, Aaberg TM, Capone A, Sternberg P et al. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 1991; 112: 647–656.
Chakravarthy U, Biggart JH, Gardiner TA, Archer DB, Maguire CJ . Focal irradiation of perforating eye injuries: minimum effective dose and optimum time of irradiation. Curr Eye Res 1989; 8: 1241–1250.
Chakravarthy U, Gardiner TA, Archer DB, Maguire CJ . A light microscopic and autoradiographic study of non-irradiated and irradiated ocular wounds. Curr Eye Res 1989; 8: 337–348.
De Gowin RL, Lewis LJ, Hoak JC, Mueller AL, Gibson DP . Radiosensitivity of human endothelial cells in culture. J Lab Clin Med 1974; 81: 42–48.
Rosander K, Zackrisson B . DNA damage in human endothelial cells after irradiation in anoxia. Acta Oncol 1995; 34: 111–116.
Hosoi Y, Yamamoto Y, Ono T, Sakamoto K . Prostacyclin production in cultured endothelial cells is highly sensitive to low doses of ionizing radiation. Int J Radiat Oncol Biol Phys 1993; 63: 631–638.
Mooteri SN, Podolski JL, Drab EA, Saclarides TJ, Onoda JM, Kantak SS . WR 1065 and radioprotection of vascular endothelial cells. II Morphology. Radiat Res 1996; 145: 217–224.
Rubin DB, Drab EA, Kang HJ, Baumann FE, Blazek ER . WR 1065 and radioprotection of vascular endothelial cells. I Cell proliferation, DNA synthesis and damage. Radiat Res 1996; 145: 210–216.
Waters CM, Taylor JM, Molteni A, Ward WF . Dose response effects of radiation on the permeability of endothelial cells in culture. Radiat Res 1996; 146: 321–328.
Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ . Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer 1997; 75: 666–672.
Archambeau JO, Mao XW, Yonemoto LT, Slater JD, Friedrichsen E, Teichman S et al. What is the role of radiation in the treatment of subfoveal membranes. Review of radiobiologic, pathologic, and other considerations to initiate a multimodality discussion. Int J Radiat Oncol Biol Phys 1998; 40: 1125–1136.
Miyamoto H, Kimura H, Yasukawa T, Honda Y, Tabata Y, Ikada Y et al. Effect of focal X-ray irradiation on experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 1999; 40: 1496–1502.
Spaide RF, Guyer DR, McCormick B, Yannuzzi LA, Burke K, Mendelsohn M et al. External beam radiation therapy for choroidal neovascularization. Ophthalmology 1998; 105: 24–30.
Postgens H, Bodanowitz S, Kroll P . Low dose radiation therapy for age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 1997; 235: 656–661.
Prettenhofer U, Haas A, Mayer R, Oechs A, Pakisch B, Stranzl H et al. Role of radiotherapy in age-related macular degeneration: a prospective study. Strahlenther Onkol 1998; 174: 613–617.
Staar S, Krott R, Mueller RP, Bartz-Schmidt KU, Heimann K . External beam radiotherapy for subretinal neovascularization in age-related macular degeneration: is this treatment efficient? Int J Radiat Oncol Biol Phys 1999; 45: 467–473.
RAD. A prospective, randomized, double-masked trial on radiation therapy for neovascular age-related degeneration (RAD Study). Radiation therapy for age-related macular degeneration. Ophthalmol 1999; 106: 2239–2247.
Marcus DM, Sheils WC, Johnson MH, McIntosh SB, Leibach DB, Maguire A et al. External beam irradiation of subfoveal choroidal neovascularization of complicating age-related macular degeneration. Arch Ophthalmol 2001; 119: 171–180.
Schittkowski M, Schneider H, Grüschow K, Ziegler PG, Guthoff R, Fietkau R . 3 Jahre Erfahrung mit der niedrig dosierten fraktionierten perkutanen Teletherapie bei subfoveolären Neovaskularisationen. Strahlenther Onkol 2001; 177: 345–353.
Bergink GJ, Deutman AF, van den Broek JF, van Daal WA, van der Maazen RW . Radiation therapy for subfoveal choroidal neovascular membranes in age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 1994; 232: 591–598.
Stalmans P, Leys A, Van Limbergen E . External beam radiotherapy (20 Gy, 2 fractions) fails to control the growth of choroidal neovascularization in age-related macular degeneration: a review of 111 cases. Retina 1997; 17: 481–492.
Eter N, Schüller H, Spitznas M . Radiotherapy for age-related macular degeneration: is there a benefit for classic CNV? Int Ophthalmol 2002; 24: 13–19.
Valmaggia C, Ries G, Ballinari P . Radiotherapy for subfoveal choroidal neovascularization in age-related macular degeneration: a randomized clinical trial. Am J Opthalmol 2002; 133: 521–529.
Yonemoto LT, Slater JD, Blacharski PB . Dose response in the treatment of subfoveal choroidal neovascularization in age-related macular degeneration: results of a Phase I/II dose escalation study using proton radiotherapy. J Radiosurg 2000; 3: 47–54.
Thoelen A, Meister A, Bernasconi PP, Messmer EP . Radiotherapie von subretinalen Neovaskularisationsmembranen bei altersabhängiger Makuladegeneration (AMD). Ophthalmologe 1998; 95: 691–698.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoeller, U., Fuisting, B., Schwartz, R. et al. Results of radiotherapy of subfoveal neovascularization with 16 and 20 Gy. Eye 19, 1151–1156 (2005). https://doi.org/10.1038/sj.eye.6701743
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701743