Abstract
Purpose
Assess short-term real-world outcomes in neovascular aged-related macular degeneration (nAMD) treated with novel faricimab.
Methods
Retrospective case series of nine patients with nAMD (11 eyes) treated with faricimab between May and November 2022. Treatment-naïve patients and non-naïve patients underwent logMAR best corrected visual acuity (BCVA), optical coherence tomography (OCT) DRI OCT-1 Triton (Topcon Corp, Tokyo, Japan), ultra-widefield (UWF) and fundus autofluorescence (FAF) (California Optomap, Optos plc, Dunfermline, Scotland, UK). Previous treatment intervals, number of intravitreal injections, sub/intra retinal fluid (SRF/IRF), central retinal thickness (CRT) and presence/changes in pigment epithelial detachments (PEDs) were recorded.
Results
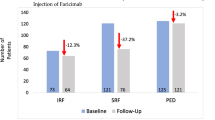
Mean baseline BCVA and CRT values of patients who switched from other agents were 0.612 ± 0.75 logMAR and 256.16 ± 12.98 µm respectively, with a mean 36-day previous treatment interval. The median number of other previous anti-VEGF intravitreal injections was 8. Mean BCVA at one month significantly improved to 0.387 ± 0.54 logMAR, as well as CRT values which decreased to 245.43 ± 15.34 µm. In the 3 naïve patients, mean baseline BVCA and CRT values were 0.33 ± 0.29 and 874.67 ± 510.86 µm, respectively. At one month follow-up, mean BCVA improved to 0.30 ± 0.29 logMAR and mean CRT was 536.04 ± 36.15 µm. Overall, a significant improvement in BCVA of 0.21 ± 41 logMAR and 238.44 ± 114.9 µm was achieved at one month after the first faricimab intravitreal injection. In addition, a complete resolution of SRF was observed in 6 out of 8 eyes (75%) and of IRF in 2 out of 3 eyes (66.67%), respectively. Drusenoid PED morphology changes were observed in all patients and no drug-related adverse events were observed.
Conclusion
Real-world outcomes showed improvement in BCVA and anatomic parameters at an early timepoint, demonstrating the efficacy and durability of faricimab in nAMD patients. Larger numbers of patients and longer follow-up are needed to determine whether the loading dose is required in all, what percentage of patients experience an improvement, and whether improvement it is maintained.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Drug Approval Package: Macugen (Pegaptanib Sodium) NDA #021756 [Internet]. [cited 2022 Nov 5]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-756_Macugen.cfm.
Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmol [Internet]. 2017;124:1296–304. https://pubmed.ncbi.nlm.nih.gov/28551167/.
Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye (Lond) [Internet]. 2020;34:802–4. https://pubmed.ncbi.nlm.nih.gov/31695160/.
Zuber-Laskawiec K, Kubicka-Trzaska A, Karska-Basta I, Pociej-Marciak W, Romanowska-Dixon B. Non-responsiveness and tachyphylaxis to anti-vascular endothelial growth factor treatment in naive patients with exudative age-related macular degeneration. J Physiol Pharm [Internet]. 2019;70:779–85. https://pubmed.ncbi.nlm.nih.gov/32009630/.
Lorés-Motta L, Riaz M, Grunin M, Corominas J, van Asten F, Pauper M, et al. Association of genetic variants with response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. JAMA Ophthalmol [Internet]. 2018;136:875–84. https://pubmed.ncbi.nlm.nih.gov/29852030/.
Ghanchi F, Bourne R, Downes SM, Gale R, Rennie C, Tapply I, et al. An update on long-acting therapies in chronic sight-threatening eye diseases of the posterior segment: AMD, DMO, RVO, uveitis and glaucoma. Eye (Lond) [Internet]. 2022;36:1154–67. https://pubmed.ncbi.nlm.nih.gov/34974541/.
Mehta H, Nguyen V, Barthelmes D, Pershing S, Chi GC, Dopart P, et al. Outcomes of over 40,000 eyes treated for diabetic macula edema in routine clinical practice: a systematic review and meta-analysis. Adv Ther [Internet]. 2022;39. https://pubmed.ncbi.nlm.nih.gov/36241963/.
Vabysmo (faricimab-svoa) FDA Approval History - Drugs.com [Internet]. https://www.drugs.com/history/vabysmo.html.
Genentech: Press Releases | Friday, Jan 28, 2022 [Internet]. https://www.gene.com/media/press-releases/14943/2022-01-28/fda-approves-genentechs-vabysmo-the-firs?sf159058256=1.
Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8:471.
Ferro Desideri L, Traverso CE, Nicolò M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin Ther Targets [Internet]. 2022;26:145–54. https://pubmed.ncbi.nlm.nih.gov/35098845/.
Sahni J, Dugel PU, Patel SS, Chittum ME, Berger B, del Valle Rubido M, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: The AVENUE phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:955–63.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40.
Eter N, Singh RP, Abreu F, Asik K, Basu K, Baumal C, et al. YOSEMITE and RHINE: phase 3 randomized clinical trials of faricimab for diabetic macular edema: study design and rationale. Ophthalmol Sci [Internet]. 2022;2:100111 http://www.ncbi.nlm.nih.gov/pubmed/36246184.
Khanani AM, Guymer RH, Basu K, Boston H, Heier JS, Korobelnik JF, et al. TENAYA and LUCERNE: rationale and design for the phase 3 clinical trials of faricimab for neovascular age-related macular degeneration. Ophthalmol Sci [Internet]. 2021;1:100076 https://pubmed.ncbi.nlm.nih.gov/36246941/.
Real world efficacy of faricimab in neovascular AMD and DME: The TRUCKEE Study – EURETINA [Internet]. https://euretina.org/resource/abstract_2022_real-world-efficacy-of-faricimab-in-neovascular-amd-and-dme-the-truckee-study/.
Nair AA, Finn AP, Sternberg P Jr. Spotlight on faricimab in the treatment of wet age-related macular degeneration: design, development and place in therapy. Drug Des Devel Ther [Internet]. 2022;16:3395–400. https://pubmed.ncbi.nlm.nih.gov/36199631/.
Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab. Curr Med Res Opin [Internet]. 2011;27:1465–75. https://pubmed.ncbi.nlm.nih.gov/21623685/.
Schmid MK, Reich O, Faes L, Boehni SC, Bittner M, Howell JP, et al. Comparison of outcomes and costs of ranibizumab and aflibercept treatment in real-life. PLoS One [Internet]. 2015;10. https://pubmed.ncbi.nlm.nih.gov/26241852/.
Vedula SS, Krzystolik MG. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev [Internet]. 2008 https://pubmed.ncbi.nlm.nih.gov/18425911/.
Yamashiro K, Oishi A, Hata M, Takahashi A, Tsujikawa A. Visual acuity outcomes of anti-VEGF treatment for neovascular age-related macular degeneration in clinical trials. Jpn J Ophthalmol [Internet]. 2021;65:741–60. https://pubmed.ncbi.nlm.nih.gov/34491474/.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica [Internet]. 2017;237:185–222. https://pubmed.ncbi.nlm.nih.gov/28423385/.
Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) [Internet]. 2015;29:1397–8. https://pubmed.ncbi.nlm.nih.gov/26446737/.
Mettu PS, Allingham MJ, Cousins SW. Incomplete response to Anti-VEGF therapy in neovascular AMD: exploring disease mechanisms and therapeutic opportunities. Prog Retin Eye Res [Internet]. 2021;82. https://pubmed.ncbi.nlm.nih.gov/33022379/.
Khanani AM, Russell MW, Aziz AA, Danzig CJ, Weng CY, Eichenbaum DA, et al. Angiopoietins as potential targets in management of retinal disease. Clin Ophthalmol [Internet]. 2021;15:3747–55. https://pubmed.ncbi.nlm.nih.gov/34511878/.
Khanani AM, Patel SS, Ferrone PJ, Osborne A, Sahni J, Grzeschik S, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol [Internet]. 2020;138:964–72. https://pubmed.ncbi.nlm.nih.gov/32729897/.
Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study: a randomized clinical trial. Retin [Internet]. 2021;41:1911–20. https://pubmed.ncbi.nlm.nih.gov/33782365/.
Viggiano P, Grassi MO, Boscia G, Pignataro M, Petruzzella G, Borrelli E, et al. Short-term morphofunctional changes in previously treated neovascular AMD eyes switched to brolucizumab. J Clin Med [Internet]. 2022 Oct [cited 2022 Dec 13];11. Available from: https://pubmed.ncbi.nlm.nih.gov/36233385/.
Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep [Internet]. 2021 Dec;11. https://pubmed.ncbi.nlm.nih.gov/33762600/.
Ogura Y, Jaffe GJ, Cheung CMG, Kokame GT, Iida T, Takahashi K, et al. Efficacy and safety of brolucizumab versus aflibercept in eyes with polypoidal choroidal vasculopathy in Japanese participants of HAWK. Br J Ophthalmol. 2022;106:994–9.
Khanani AM, Zarbin MA, Barakat MR, Albini TA, Kaiser PK, Guruprasad B, et al. Safety outcomes of brolucizumab in neovascular age-related macular degeneration: results from the IRIS Registry and Komodo Healthcare Map. JAMA Ophthalmol [Internet]. 2022;140:20–8. https://pubmed.ncbi.nlm.nih.gov/34817566/.
Baumal CR, Sørensen TL, Karcher H, Freitas RL, Becher A, Balez S, et al. Efficacy and safety of brolucizumab in age-related macular degeneration: a systematic review of real-world studies. Acta Ophthalmol [Internet]. 2022 [cited 2022 Nov 9]; Available from: https://pubmed.ncbi.nlm.nih.gov/36117281/.
Saenz-de-Viteri M, Recalde S, Fernandez-Robredo P, López Gálvez MI, Arias Barquet L, Figueroa MS, et al. Role of intraretinal and subretinal fluid on clinical and anatomical outcomes in patients with neovascular age-related macular degeneration treated with bimonthly, treat-and-extend and as-needed ranibizumab in the In-Eye study. Acta Ophthalmol [Internet]. 2021;99:861–70. https://pubmed.ncbi.nlm.nih.gov/33720541/.
Shijo T, Sakurada Y, Tanaka K, Miki A, Sugiyama A, Onoe H, et al. Incidence and risk of advanced age-related macular degeneration in eyes with drusenoid pigment epithelial detachment. Sci Rep [Internet]. 2022 Dec [cited 2022 Nov 28];12. Available from: https://pubmed.ncbi.nlm.nih.gov/35304557/.
Javaheri M, Hill L, Ghanekar A, Stoilov I. Changes in treatment-naive pigment epithelial detachments associated with the initial anti-vascular endothelial growth factor injection: a post hoc analysis from the HARBOR trial. JAMA Ophthalmol [Internet]. 2021;139:219–23. https://pubmed.ncbi.nlm.nih.gov/33331859/.
Rispoli M, Eandi CM, di Antonio L, Kilian R, Montesel A, Savastano MC. Biomarkers in early response to brolucizumab on pigment epithelium detachment associated with exudative age-related macular degeneration. Biomedicines [Internet]. 2021 Jun [cited 2022 Nov 8];9. Available from: https://pubmed.ncbi.nlm.nih.gov/34200829/.
Mathews JP, Jalil A, Lavin MJ, Stanga PE. Retinal pigment epithelial tear following intravitreal injection of bevacizumab (Avastin): optical coherence tomography and fluorescein angiographic findings. Eye (Lond). 2007;21:1004–5.
Hussain RM, Neiweem AE, Kansara V, Harris A, Ciulla TA. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs [Internet]. 2019;28:861–9. https://pubmed.ncbi.nlm.nih.gov/31513439/.
Collazos-Alemán JD, Gnecco-González S, Jaramillo-Zarama B, Jiménez-Mora MA, Mendivil CO. The role of angiopoietins in neovascular diabetes-related retinal diseases. Diabetes Ther [Internet]. 2022 Nov [cited 2022 Nov 8]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/36331711.
Khan M, Aziz AA, Shafi NA, Abbas T, Khanani AM. Targeting angiopoietin in retinal vascular diseases: a literature review and summary of clinical trials involving faricimab. Cells. 2020;9:1869.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design, writing, critical review and approval the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stanga, P.E., Valentín-Bravo, F.J., Stanga, S.E.F. et al. Faricimab in neovascular AMD: first report of real-world outcomes in an independent retina clinic. Eye 37, 3282–3289 (2023). https://doi.org/10.1038/s41433-023-02505-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02505-z
This article is cited by
-
Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME
International Journal of Retina and Vitreous (2024)
-
One-year visual and anatomical outcomes of intravitreal faricimab injection for neovascular age-related macular degeneration after prior brolucizumab treatment
Scientific Reports (2024)
-
Intravitreal faricimab for neovascular age-related macular degeneration previously treated with traditional anti-VEGF compounds: a real-world prospective study
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Efficacy, Durability and Safety of Faricimab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Oedema: Lessons Learned from Registration Trials
Ophthalmology and Therapy (2023)