Abstract
Purpose
To evaluate the safety and efficacy of surgical implantation of prosthetic iris devices in patients with iris deficiency.
Methods
Nine patients with traumatic iris defects, congenital aniridia or iris coloboma, and surgical or optical iridectomies were included in a noncomparative case series. Cataract surgery with intraocular lens and prosthetic iris implantation was performed in 10 eyes. The visual acuity, subjective degree of glare disability, postoperative anatomic results, and intraoperative and postoperative complications were evaluated.
Results
The mean follow-up was 17.75 months (range 4–48 months). Best-corrected visual acuity improved in nine of 10 eyes (90%) and remained unchanged in one eye. Glare subjectively improved in four of five eyes (80%) of patients complaining of glare preoperatively. All eyes achieved the desired anatomic result. Intraoperative complications included one anterior capsular tear. Postoperative complications included a short period of mild postoperative anterior uveitis in four eyes. Secondary glaucoma was absent.
Conclusion
In patients with iris deficiency, implantation of prosthetic iris device, and intraocular lens implant following cataract surgery appears to be safe and effective in reducing glare disability and improving visual outcomes.
Similar content being viewed by others
Introduction
Iris deficiency can be congenital or traumatic. The congenital iris deficiency-associated findings that contribute to reduced visual acuity are corneal pannus and epitheliopathy, glaucoma, foveal and optic nerve hypoplasia, cataract, and nystagmus.1 Traumatic iris deficiency is often accompanied with corneal scarring, cataract, and secondary glaucoma. Symptoms of iris deficiency range from decreased visual acuity and poor cosmetic appearance to glare, and photophobia. Various methods have been used to overcome the disabling effects of iris deficiency, including eyelid surgery, coloured contact lenses,2, 3 corneal tattooing,4, 5 and implantation of artificial irides.6, 7, 8, 9, 10, 11, 12, 13, 14
In 1994, Sundmacher and coauthors were the first to report the use of a black iris-diaphragm posterior chamber intraocular lens (IOL) to correct congenital and traumatic aniridia.6, 7, 8 These prosthetic implants have evolved over the last decade. The purpose of the study was to evaluate the safety and efficacy of surgical implantation of prosthetic iris devices in patients with iris deficiency.
Materials and methods
This was a retrospective noncomparative case series from 1998 to 2002. It includes nine consecutive patients (10 eyes) with iris deficiency, who were treated at the Sussex Eye Hospital, Brighton and Sussex University Hospitals, Brighton, the Eye Unit, Pembury Hospital, Royal Tunbridge Wells, and the Department of Ophthalmology, Eastbourne District General Hospital, Eastbourne, UK.
Of the nine patients who have been studied, six are male and three are female. The age range varied between 31 and 80 years with a mean age of 53.8 years. Four of the iris deficiencies were traumatic (three total and one sectorial iris loss), four were iatrogenic [three optical iridectomies (previous treatment for congenital cataract), and one surgical iridectomy to remove a malignant melanoma of the iris], and two were congenital (one aniridia and one coloboma).
Inclusion criterion was any patient with partial or total iris deficiency, and exclusion criterion was postoperative follow-up of less than 4 months.
Preoperative ophthalmologic assessment included Snellen best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure measurement using Goldmann applanation tonometry, gonioscopy, and fundoscopy following pupillary dilation when feasible.
Each eye underwent cataract surgery (eight phaco, one ECCE) with IOL and prosthetic iris device implantation.
The visual acuity, subjective degree of glare disability, postoperative anatomic results and intraoperative and postoperative complications were evaluated (Table 1).
In particular, the subjective degree of glare disability was evaluated pre- and postoperatively with the use of a questionnaire that graded glare as follows: no glare, mild glare, moderate glare, and severe glare.
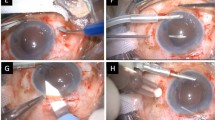
The prosthetic iris devices used in this study are the Morcher aniridia IOL Types 67F and 67G, the aniridia ring Type 50C, and the coloboma diaphragm Type 96G (Figure 1). Types 67F and 67G are biconvex poly(methyl methacrylate) (PMMA) IOLs with an overall (haptic) diameter of 13.5 and 12.5 mm respectively, and a diaphragm diameter of 10 mm. The periphery of the diaphragm is treated with a black co-polymer leaving a central 5.0 mm transparent.
Type 50C has an overall diameter of 10.75 mm and two of these rings have to be implanted and turned against each other in order to produce a full iris diaphragm. Type 96G has an overall diameter of 11.0 mm. Types 67G and 67F have been designed to be inserted through a corneal incision of at least 11 mm, while Types 50C and 96G are inserted through a small incision.
Results
The mean follow-up was 17. 75 months (range 4–48 months). Nine eyes underwent phacoemulsification and one extracapsular cataract extraction. In six eyes a 96G implant was inserted (Figure 2). Insertion of an additional capsular tension ring was performed in one of the above eyes. Two 50C implants were used in two eyes. Both the 96G and 50C implants were inserted into the capsular bag through a small incision. The incision was enlarged to accommodate endocapsular implantation of a 67G IOL in one eye (Figure 3). A 67F IOL was introduced in the ciliary sulcus in the patient that underwent extracapsular cataract extraction.
Best-corrected visual acuity improved in nine of 10 eyes (90%), with an average of three lines on the Snellen chart. In one eye the visual acuity remained unchanged. This was in the patient with congenital aniridia, whose visual potential was limited by nystagmus. Glare subjectively improved in four of five eyes (80%) of patients complaining of glare preoperatively. In the remaining eye the degree of glare disability was the same pre- and postoperatively. No glare was reported before or after surgery in three eyes, and two patients did not return their completed questionnaire.
Intraoperative complications included one anterior capsular tear, during implantation of a 50C ring. Postoperative complications included a short period of mild postoperative anterior uveitis in four eyes. Secondary glaucoma was absent in all cases. Eight of 10 prosthetic iris devices remained well centred throughout the follow-up period. In one eye the 96G implant migrated centrally secondary to capsular contraction (Figure 4). In the eye with the anterior capsular tear, the 50C rings migrated in the sulcus, and misaligning of the ring implants also occurred (Figure 5).
Posterior capsular opacification occurred in one eye with congenital aniridia, cataract, and posterior lenticonus 4.5 months postoperatively, requiring YAG laser capsulotomy.
Discussion
In the current literature,6, 7, 8, 9, 10, 11, 12, 13, 14 the most commonly used prosthetic iris devices are the ones used in our study. Namely, the Morcher aniridia IOL Types 67F and 67G, the aniridia ring Type 50C, and the coloboma diaphragm Type 96G.
Types 67F and 67G are used in traumatic or congenital aniridia either in the ciliary sulcus, if there is adequate capsular support, or trans-sclerally sutured in the absence of capsular support.6, 7, 8, 9, 10, 11, 12, 13 We were the first to report implantation of a Morcher 67G aniridia IOL within the capsular bag.14 Type 50C is also designed for endocapsular implantation in cases of traumatic or congenital aniridia.11, 13 For a total reconstruction of the missing iris, two rings have to be implanted and turned against each other to form a complete ring, and then an additional foldable lens is inserted. Type 96G is used in the capsular bag in cases of sector iris defects up to 90°.11, 13 Iris defects between 90° and 180° could be managed by inserting two such devices.
Advantages of endocapsular over sulcus or trans-sclerally sutured aniridia IOLs may include reduction in the incidence of glaucoma and improvement of the stability of the device, provided the capsular bag is intact.14 The main disadvantage of the use of a single-piece iris black diaphragm and optical lens is the requirement for large incision of at least 10 mm. Moreover, they are brittle, like all blackened PMMA devices. Alternatively two capsular tension rings with iris diaphragm (Morcher, Type 50C) can be introduced without having to enlarge the small incision in phacoemulsification procedures, as these devices do not have an optical portion. The two rings are then assembled within the eye, followed by implantation of a foldable lens. However, the disadvantages of the 50C aniridia rings are that these devices are also brittle, difficult to align within the eye in order to produce a full iris diaphragm, and even if this is achieved intraoperatively, there is a possibility of them misaligning long after they have been implanted into the aniridic eye with contracture of the capsular bag.14 Finally, the capsular bag can become somewhat crowded after three devices have been inserted.11
Alternatively, one can use prosthetic iris devices produced by Opthec. There is a wide variety and some of their designs can be used in combination. Additional considerations include a variety of pupil diameters and availability in four different colours namely black, brown, blue, and green.
In our study, the visual acuity and subjective degree of glare disability improved in 90% and 80% (the other 20% unchanged) of the cases respectively. While cataract removal usually reduces glare, this is not the case in the presence of iris deficiency as the optic diameter of an IOL is much smaller than that of the natural lens and would leave the area of iris deficiency without optical power, creating more glare. Improvement of glare in 80% of the cases is therefore an excellent result.
Complications included one anterior capsular tear, possibly due to abnormal capsular fragility associated with congenital aniridia. This was further complicated by migration of both Type 50C devices into the ciliary sulcus. In one eye the 96G implant migrated centrally secondary to capsular contraction. The same patient underwent identical surgery to the fellow eye, and to avoid the same complication a Type 14 Morcher capsular tension ring was also inserted. The implant remained well-centred and no migration was noted. It is therefore of utmost importance to achieve a perfect and well centred capsulorrhexis, to avoid decentration and migration of devices and IOLs outside the capsular bag.
There was a short period of mild postoperative anterior uveitis in four eyes. The subject of postoperative inflammation varies widely within the literature. In some reports slight persistent intraocular inflammation was noted,6, 7, 8, 12 in some others there was a short period of mild postoperative anterior uveitis,9, 14 and in others no inflammation was present.10, 11
Secondary glaucoma was absent in all cases. However, glaucoma is another postoperative complication that is reported in other studies.6, 7, 8, 9, 12, 13 Reinhard et al12 in their latest study suggest that apart from the fact that patients with aniridia have increased risk for developing glaucoma, the blood–aqueous barrier (BAB) is also altered, mainly because of the implantation of the black diaphragm aniridia IOL in front of the capsular bag, accelerating glaucoma progression. Possible reasons for chronic alteration of the BAB include direct contact of the IOL with uveal remnants, ‘dislocated’ haptics in the angle of the anterior chamber, and a more sensitive BAB, to all types of trauma, in eyes with aniridia.12 However, all the above-mentioned risk factors can be effectively eliminated if the IOL is implanted within the capsular bag, when feasible, as we did in our patients.
In conclusion, we consider that in patients with iris deficiency, implantation of prosthetic iris device, and intraocular lens implant following cataract surgery appears to be safe and effective in reducing glare disability, thus improving the visual outcome.
Further evolution of artificial irides in association with increasing experience using these devices will result in overall improvement of the surgical outcomes. Additional design considerations may include custom pupil size, custom iris colour, and improved flexibility of the material. As breakage can happen readily, we advocate ordering a spare device.
References
Eagle Jr RC . Congenital, developmental, and degenerative disorders of the iris and ciliary body. In: Albert DM, Jacobiec FA (eds). Principles and Practice of Ophthalmology. WB Saunders: Philadelphia, 2000, pp 1151–1153.
Burger DS, London R . Soft opaque contact lenses in binocular vision problems. J Am Optom Assoc 1993; 64: 176–180.
Schulze F . Irisrekonstruction: operation, laser order kontaktlinsen mit irisstruktur. Fortschr Ophthalmol 1991; 88: 30–34.
Beekhuis WH, Drost BHIM, Velden EM van der Samderubun . A new treatment for photophobia in posttraumatic aniridia: a case report. Cornea 1998; 17: 338–341.
Burris TE, Holmes-Higgin DK, Silvestrini TA . Lamellar intrastromal corneal tattoo for treating iris defects (artificial iris). Cornea 1998; 17: 169–173.
Sundmacher R, Reinhard T, Althaus C . Black-diaphragm intraocular lens for correction of aniridia. Ophthalmic Surg 1994; 25: 180–185.
Reinhard T, Sundmacher R, Althaus C . Irisblenden-IOL bei traumtischer aniridie. Klin Monatsbl Augenheilkd 1994; 205: 196–200.
Sundmacher R, Reinhard T, Althaus C . Black diaphragm intraocular lens in congenital aniridia. Ger J Ophthalmol 1994; 3: 197–201.
Thompson CG, Fawzy K, Bryce IG, Noble BA . Implantation of a black diaphragm intraocular lens for traumatic aniridia. J Cataract Refract Surg 1999; 25: 808–813.
Tanzer DJ, Smith RE . Black iris-diaphragm intraocular lens for aniridia and aphakia. J Cataract Refract Surg 1999; 25: 1548–1551.
Osher RH, Burk SE . Cataract surgery combined with implantation of an artificial iris. J Cataract Refract Surg 1999; 25: 1540–1547.
Reinhard T, Engelhardt S, Sundmacher R . Black diaphragm aniridia intraocular lens for congenital aniridia: long-term follow-up. J Cataract Refract Surg 2000; 26: 375–381.
Burk SE, Da Mata AP, Snyder ME, Cionni RJ, Cohen JS, Osher RH . Prosthetic iris implantation for congenital, traumatic, or functional iris deficiencies. J Cataract Refract Surg 2001; 27: 1732–1740.
Mavrikakis I, Hickman Casey JM . Phacoemulsification and endocapsular implantation of an artificial iris intraocular lens in traumatic catatract and aniridia. J Cataract Refract Surg 2002; 28: 1088–1091.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in this study. There was no financial support. Presented in part at the annual meeting of the Royal College of Ophthalmologists Birmingham, May 2003.
Rights and permissions
About this article
Cite this article
Mavrikakis, I., Mavrikakis, E., Syam, P. et al. Surgical management of iris defects with prosthetic iris devices. Eye 19, 205–209 (2005). https://doi.org/10.1038/sj.eye.6701448
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701448
Keywords
This article is cited by
-
Chronic post-operative iris prosthesis endophthalmitis in a patient with traumatic aniridia: a case report
BMC Ophthalmology (2016)
-
Long-term results after artificial iris implantation in patients with aniridia
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Irisprothetik
Der Ophthalmologe (2011)