Abstract
Disruption of synaptic integrity, loss of connectivity and axodendritic degeneration are early and essential components of neurodegeneration. Although neuronal cell death mechanisms have been thoroughly investigated, less is known about the signals involved in axodendritic damage and the processes involved in regeneration. Here we conducted a genome-wide RNA interference-based forward genetic screen, using small interfering RNA targeting all human kinases, and identified clusters of kinases families essential for growth cone collapse, neurite retraction and neurite outgrowth. Of 59 kinases identified as positive regulators of neurite outgrowth, almost 50% were in the tyrosine kinase/tyrosine kinase-like (TK/TKL) receptor subgroups, underlining the importance of extracellular ligands in this process. Neurite outgrowth was inhibited by 66 other kinases, none of which were TK/TKL members, whereas 79 kinases inhibited lysophosphatidic acid-induced neurite retraction. Twenty kinases were involved in both inhibitory processes suggesting shared mechanisms. Within this group of 20 kinases, some (ULK1, PDK1, MAP4K4) have been implicated previously in axonal events, but others (MAST2, FASTK, CKM and DGUOK) have not. For a subset of kinases, the effect on neurite outgrowth was validated in rat primary cerebellar cultures. The ability to affect regeneration was further tested in a model of axodendritic lesion using primary rat midbrain cultures. Finally, we demonstrated that haploinsufficiency of two members of the AGC kinase subgroup, ROCK1 and PKN1, was able to suppress retinal degeneration in Drosophila model of class III Autosomal Dominant Retinitis Pigmentosa.
Similar content being viewed by others
Main
Neurodegenerative diseases, such as Alzheimer's, Parkinson's and Huntington's, are characterized by neuronal degeneration in the central nervous system (CNS). Research efforts have focused mainly on neuronal demise, of which apoptosis, necrosis and autophagic death of the neuronal soma are the most commonly described.1 On the contrary, the disruption of synaptic integrity, loss of connectivity and axonal degeneration which often precede, and are not always associated with, neuronal demise have not been thoroughly addressed. Although the mechanisms leading to axonal degeneration are largely unknown, there is compelling evidence that different execution systems operate in the axons and in the soma during neuronal degeneration.2, 3, 4 Selective, localized processes are also critical for neurite sprouting, axon growth, extension of the dendritic tree and establishment of precise connections with specific target cells. These are active during neuronal development, but they are also important for neuronal plasticity, as well as neuronal regeneration after damage to neurites and nerve endings.
Processes of both neuronal degeneration and regeneration involve complex signalling cascades leading to remodelling of cytoskeletal architecture, changes in ion signalling and mitochondrial function and distribution. Several signalling cascades involved in neurodegenerative/regenerative processes involve protein phosphorylation by kinases.5, 6 Aberrantly active protein kinases have been implicated in a variety of disease states, including neurodegenerative disorders7, 8 and kinases are directly responsible for some of the most characteristic neuronal alterations in neurodegeneration. For example, CDK5 and GSK3β regulate hyperphosphorylation of the microtubule-associated protein tau and formation of intraneuronal neurofibrillary tangles (NFTs) in Alzheimer's disease (AD) patients.9, 10 Overexpression of CDK5 and GSK3β in neuronal cultures and transgenic animal models not only leads to tau phosphorylation but also causes cytoskeletal structural abnormalities and neurodegeneration.11 Recent studies indicate that the genes encoding the PINK1 and LRRK2 protein kinases are mutated in patients with early and late onset Parkinson's disease, respectively12, 13, 14, 15, 16 and work in Drosophila melanogaster has provided further evidence of the role of mutant PINK1 in mitochondria dysfunction and cell death.17 Altered kinase activity is essential for the neurodegenerative effects of mutant PINK1 and LRRK2. Pathogenic mutants and kinase-dead PINK1 abrogate the neuroprotective effect of wild-type PINK1,18 whereas pathogenic mutations in LRRK2 increase its kinase activity, and cause neurodegeneration in neuronal cultures.19, 20, 21, 22 Several other kinases, such as members of the casein kinases family (CK1δ, CK1α) and DYRK1A, have also been implicated in tauopathies such as AD, based on their abilities to phosphorylate tau, as well as β-secretase and presenilin, two other proteins linked to AD.23, 24
Despite the enormous research focus on individual pathways involving kinase regulation in neurodegenerative disorders, the underlying consequences of a disturbed signalling machinery are still obscure. Most importantly, although the position of individual kinases in signal transduction is well established in many cases, there is no comprehensive picture of the overall cross-talk between kinase families in pathophysiological processes. To address this complexity, we designed an RNA interference (RNAi)-based forward genetic screen, using small interfering RNA (siRNA) targeting the majority of human kinases, with the aim of identifying clusters of kinases essential for growth cone collapse, neurite retraction and neurite outgrowth. In addition to unraveling the complex interactions between different kinase families, this study has also uncovered novel roles for kinases that have not yet been assigned a function or have not previously been implicated in neuronal function. This may foster new understanding of mechanisms involved in neurodegeneration and help in designing effective and non-toxic treatments based on modulation of different kinase subsets.
Results
High-throughput screen of human kinases involved in neurite outgrowth
To establish the role of protein kinases in neurite outgrowth and retraction, we designed a functional assay using the human neuroblastoma cell line, SH-SY5Y. In the presence of dibutyryl-cyclic AMP (db-cAMP), SH-SY5Y cells form elongated β-III tubulin-immunopositive processes within 48 h (Figure 1a and b). The average neurite length per neuron (ANL/neuron) for non-differentiated and differentiated cells was 40–50 and 70–80 μm, respectively (Figure 1e). SH-SY5Y cells were then systematically transfected with siRNAs using a kinome library comprising 750 kinases at three-fold redundancy. Cells were then differentiated in medium containing db-cAMP (Supplementary Figure 1). Under these conditions, more than 92% of cells were transfected with siRNA (Supplementary Figure 2). As selection criterion, we used neurite length and considered a kinase to be genuine when two or more siRNA against the same candidate kinase produced the same phenotype (low or high ANL/neuron). Because undifferentiated SH-SY5Y cells undergo cell division, siRNAs to some kinases also affected their proliferation rate and thereby might have affected only indirectly their neurite outgrowth (Figure 1g). However, some cell cycle kinases were also involved in neurite outgrowth suggesting possible new functions for this kinase family. After two rounds of stringent analyses, siRNAs (two or more) that increase the ANL/neuron to >100 μm were defined as promoting neurite outgrowth and those that decrease the ANL/neuron to <70 μm were defined as inhibiting neurite outgrowth (Figure 1f).
The high throughput neurite outgrowth assay. SH SY-5Y cells were fixed after 48 h in non-differentiating (a and c) or differentiating (b and d) medium and immunostained with neuronal-specific marker βIII tubulin. Fluorescence images (with 10 × magnification) acquired using the Cellomics High Content system (a and b) and the same images following application of neurite outgrowth algorithm (c and d). (e) A histogram showing the average neurite length/neuron of SH SY-5Y cells in differentiating or non-differentiating medium 48 h post-transfection with scrambled siRNA. Error bars=S.D., n=40 in all groups, *P value=1.98 × 10−35 with one tailed t-test. (f) A histogram showing the average neurite length/neuron of all samples (750 kinases siRNA with three-fold redundancy). Candidates with average neurite length/neuron less than 70 μm and more than 100 μm were highlighted in green and red, respectively. (g) A scatter plot showing the distribution of total numbers of neuron acquired and their respective calculated average neurite length per neuron
High-throughput screen of human kinases involved in neurite retraction
In the neurite retraction assay, db-cAMP-differentiated cells were treated with lysophosphatidic acid (LPA) for 1 h to induce growth cone collapse and neurite retraction (Figure 2b). As a positive control, we used siRNA to Rock1, which is known to inhibit these processes (Katoh et al.25 and Figure 2c). In addition, LPA induction also caused the cell body to flatten (Figure 2b and c). In this screen, the ANL/neurite for differentiated cells with or without LPA induction was 10.18 and 13.58 μm, respectively (Figure 2g). As for the outgrowth screen, the entire retraction screen was repeated and an average of both experiments were analysed. siRNAs (two or more) that maintained ANL/neurite to >12 μm were defined as inhibiting LPA-induced neurite retraction (Figure 2h).
The high throughput neurite retraction assay. SH SY-5Y cells were fixed 48 h post-transfection with scrambled siRNA (a, b, d and e) or ROCK1 siRNA (c and f) in differentiating medium and immunostained with neuronal-specific marker βIII tubulin. Fluorescence images (with × 10 magnification) acquired using the Cellomics High Content system (a, b and c) and the same images following application of extended neurite Outgrowth algorithm (d, e and f). (g) A histogram showing the average neurite length/neurite of SH SY-5Y cells in differentiating medium, with or without 10 μM LPA induction, 48 h post-transfection with scrambled siRNA. Error bars=S.D., n=104 in all groups, *P value=9.0 × 10−42 with one tailed t-test. (h) A histogram showing the average neurite length/neurite of all samples (750 kinases siRNA with three-fold redundancy). Candidates with average neurite length/neurite more than 12 μm were defined as inhibiting LPA-induced neurite retraction. (i) A scatter plot showing the distribution of total numbers of neuron acquired and their respective calculated average neurite length per neurite
Analysis of the screen data
Our analysis revealed that siRNA-targeted downregulation of ∼8% (59 candidates) of the kinome library resulted in neuronal cells exhibiting short neurite length (Table 1). In the case of 13 kinases, neurite outgrowth was inhibited by all three independent siRNAs. The efficiency of downregulation by all three siRNAs for four candidates (SNARK1, EPHA1, ERBB2 and JAK1) was further confirmed by quantitative RT-PCR (Supplementary Figure 3). Interestingly, inhibition of neurite outgrowth by LIMK1 was observed from only two independent siRNAs and this was due to an inefficient downregulation by the third siRNA (Supplementary Figure 3). Targeted downregulation of another 9% (66) kinases resulted in cells with abnormally long neurite length (Table 2). In the case of 15 kinases, neurite outgrowth was promoted by all three independent siRNAs.
The processes of growth cone collapse and neurite retraction induced by LPA were inhibited by 79 kinases. Interestingly, 20 of these kinases were identical to those which inhibited neurite outgrowth (Table 3 and Figure 3), suggesting a shared mechanism in the inhibition of outgrowth and retraction. The kinases identified in all three data sets are clustered in the kinome (http://kinase.com). The majority (50%) of the positive modulators of neurite outgrowth (inhibitory candidates) belong to the tyrosine kinase (TK) and tyrosine kinase-like (TKL) subgroups (Table 1 and Figure 3). This finding suggests that neurite outgrowth is promoted primarily by extracellular ligands and growth factors and suggests that this subset of kinases could be a potential target for therapeutic intervention. However, on the contrary, most of the negative modulators (stimulatory candidates) of neurite outgrowth are from the AGC (containing protein kinase A (PKA), protein kinase G (PKG), protein kinase C (PKC) families) subgroup and no TK/TKL kinases are involved in this process (Table 2 and Figure 3). Promotion and inhibition of neurite outgrowth required signalling from different subgroups of kinase families. Our results also revealed that the control of neurite length required a balance of cellular signals that both promotes and restricts neurite outgrowth. By contrast, no kinases that promote neurite outgrowth were involved in LPA-induced neurite retraction. The 27 TK/TKL-positive modulators of neurite outgrowth were different from the 19 TK/TKL kinases involved in the retraction, suggesting that distinct and non-over-lapping signalling mechanisms are responsible for these two processes.
Candidate kinases identified in neurite outgrowth and retraction screens. All candidate kinases identified in both the neurite outgrowth and retraction screens are listed. (a) A Venn diagram showing RNAi downregulation of candidates in yellow or red circles resulted in inhibition or promotion of neurite outgrowth, respectively. RNAi downregulation of candidates in blue circles inhibits neurite retraction induced by LPA induction. Note that there are overlapping candidates between these data sets. All candidates were colour coded according to their subgroups classification in the KINOME (http://kinase.com/kinbase/). TK, tyrosine kinase in grey; TKL, tyrosine kinase-like in blue; STE, homologues of yeast sterile 7, sterile 11, sterile 20 kinases in brown; ATYPICAL, ‘atypical’ kinases with no sequence similarity to typical kinases but are known or predicted to have enzymatic activity in turquoise; AGC, containing PKA, PKG, PKC families in green; CAMK, calcium/calmodulin-dependent protein kinase in pink; CMGC, containing CDK, MAPK, GSK3, CLK families in orange; UNCLASSIFIED, candidates present in the library but could not be linked to a particular subgroup in the KINOME in purple; OTHER, the other group contains several kinase families that do not fit within any of the other main kinase groups in red. (b) The kinase signalling cross-talk, and connection between different members of kinases families identified in the screens. Links were established between identified kinases using PathwayArchitectTM software (Stratagene). For clarity, the pathway was generated automatically by a proprietary algorithm that finds direct connections between selected candidate kinases within the context of hand-curated and text-mined data present in the database. Candidates with rhombus or ellipse shapes are from the inhibition or promotion list of the outgrowth screen, respectively. Candidates with a rectangular shape are from the list of the inhibition of LPA-induced neurite retraction and those in octagon shape overlapped between promoting list and retraction list. Colour coding of kinases subgroup classification are as in (a). Small green squares linking two nodes represent positive or negative regulation as indicated. Small blue squares and orange circles represent binding interaction and protein modification between two nodes, respectively
Validation of the screens in primary neurons
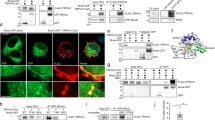
To validate the authenticity of our screen results, we selected a subset of siRNA to downregulate kinases in primary cultures of cerebellar granule neurons (CGN). Two independent siRNAs to rat orthologues of six candidates (cdc2a, ulk1, mast2, fastk, pdk1 and map4k4), identified by the promoting outgrowth data set, were co-transfected with a green fluorescent protein (GFP) reporter construct and fluorescence images of siRNA-transfected cultures were acquired 2 days post-transfection (Figure 4a–d). The outgrowth of neuronal processes from GFP-positive neurons was quantified and compared to cultures transfected with scrambled siRNA. The neurite length/neuron values were significantly higher than those with scrambled siRNA control for all CGN cultures (Figure 4).
Neurite outgrowth of CGN cultures after siRNA-mediated knockdown. Representative fluorescent images of culture CGN co-transfected with pmaxGFP and scrambled siRNA (a) or siRNA-targeting MAST2 (b), ULK1 (c) and FASTK (d). Neurite length was traced on CGN population (arrows) but not on the distinctive larger population of glial cells (arrowhead). Scale bar=50 μm. (e) A scatter plot of total neurite length per neuron for all sample traced. Each dot represents a traced neuron and the black bar represents the average neurite length per neuron (μm) for each sample. siRNA-mediated knock down of selected kinases result in significantly longer total neurite length per neuron than the scramble siRNA, using the two-tailed t-test: P=0.00039 (cdc2a siRNA1), P=0.000014 (cdc2a siRNA2), P=0.0018 (ulk1 siRNA1), P=0.00013 (ulk1 siRNA2), P=0.00089 (mast2 siRNA1), P=0.007 (mast2 siRNA2), P=0.0011 (fastk siRNA1), P=0.0258 (fastk siRNA2), P=0.0058 (pdk1 siRNA1), P=0.0348 (pdk1 siRNA2), P=0.000005 (map4k4 siRNA1) and P=0.00024 (map4k4 siRNA2)
The regeneration data were validated in another subset of kinases using a model of primary dopaminergic neuron regeneration.26 Axons were transected at day in vitro (DIV) 3 and siRNA-transfected cultures were then kept until DIV 6 to allow for regeneration of lesioned axons. Regeneration from the edge of the transection was then quantified and compared to cultures transfected with a control-siRNA-targeting enhanced green fluorescent protein (EGFP), where the length of regenerating neurites was set to 100% (Figure 5b). Regeneration in the overall culture, containing a majority of GABAergic cells and up to 10% dopaminergic neurons, was quantified in phase contrast images. A significant increase in the length of regenerating neurites was observed in cultures treated with siRNAs directed against CKM, FASTK, MAP4K4, ULK1, MAST2 and PDK1 (Figure 5a). The most pronounced effect on neurite regeneration was observed after knockdown of ULK1 (167±12%), PDK1 (165±15%) and FASTK (163±19%) compared to the anti-EGFP siRNA-treated controls (100±8%).
Regeneration of primary midbrain neurons after RNAi-induced knockdown. Bar diagrams represent quantification of neurite regeneration beyond the scratch lesion in the total knockdown culture given in percent compared to cultures treated with anti-EGFP siRNA; (a) regeneration in total culture, (c) regeneration in the dopaminergic sub-population. Data are given as mean±S.E.M. Neurite lengths were considered significantly different at *P<0.05, **P<0.01 and ***P<0.001 according to a two-sided t-test compared with the anti-EGFP siRNA-treated control. Photomicrographs: representative phase contrast (b) or epifluorescence images (d; blue – DAPI, green – TH) displaying regenerating neurites beyond the scratch lesion in cultures treated with siRNA against selected kinases or EGFP. Bar: 100 μm
To quantify regeneration in the sub-population of dopaminergic neurons in culture, we performed immunocytochemistry for tyrosine hydroxylase (TH) and measured regeneration beyond the edge of the transection as in the total culture (Figure 5d). In general, regeneration of dopaminergic neurons was less pronounced compared to overall regeneration in culture, suggesting a lower regeneration potential of this sub-population (Figure 5c). Increased regeneration of TH-positive neurons was observed with knockdown of DGUOK (131±9%), MAP4K4 (124±6%) and ULK1 (130±8%) compared to the anti-EGFP siRNA-treated control.
Validation in Drosophila retinal degeneration
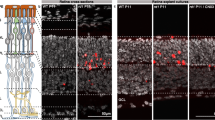
To address the physiological significance of our finding in vivo, we tested Drosophila orthologues of two candidate kinases essential for neurite retraction, ROCK1 and PKN1, for their ability to influence retinal degeneration in class III Autosomal Dominant Drosophila Retinitis Pigmentosa (ADRP). We used two mutant alleles of Drosophila ninaE gene, ninaEG69D and ninaERH27, which share molecular and phenotypic characteristics identical to those found in human ADRP.27, 28 Heterozygous ninaE flies exhibit age-related retinal degeneration.28 The severity of degeneration intensified when the animals were reared in permanent light and to a lesser extent when reared in darkness29 (Figure 6a and b). We tested whether mutant alleles of the fly orthologue of human ROCK1, rok, could influence light-induced retinal degeneration in ninaE flies. Heterozygous rok mutant suppressed retinal degeneration in ninaE heterozygous background (Figure 6a). We also tested another candidate kinase from the retraction screen, PKN1, using this light-induced retinal degeneration assay. Similarly, heterozygous pkn mutant suppressed retinal degeneration in ninaE flies, although to a lesser extent than those exhibited by rok mutant (Figure 6b).
Haploinsufficiency of ROK and PKN1 suppressed ninaE−/+ retinal degeneration. Light micrographs of retinal tangential sections of flies: (a) ninaEG69D−/+, rokf04065−/+; ninaEG69D−/+; (b) ninaERH27−/+, pknrG232−/+; ninaERH27−/+ raised in constant darkness or constant light (2000 lux) for 4 weeks. Light-induced retinal degeneration in two genetic backgrounds of ninaE mutations, ninaEG69D and ninaERH27 were suppressed by haploinsufficiency of ROK (rokf04065) and PKN (pknrG232). Upper panels of both (a) and (b) are × 40 magnification, whereas the lower panels are of × 100 magnification
Discussion
A comprehensive understanding of the molecular modulators and signalling network involved in the processes of axon outgrowth, growth cone collapse, axon retraction and degeneration is fundamental in order to gain insight into the mechanism of neuronal degeneration and regeneration. Our genome-wide screen provides a comprehensive analysis and classification of kinase gene families on the basis of their role in neurite outgrowth and retraction. This study not only places known players of neurite outgrowth and retraction into a wider context, but also identifies a large group of kinases with previously unknown function or kinases not previously implicated in regulation of axonal growth/maintenance.
Our results show that approximately 8 and 9% of the whole KINOME is essential for the promotion and inhibition of neurite outgrowth, respectively. As expected, known kinase regulators of mammalian axonal outgrowth, such as TRKB, and members of the Eph receptor TK family and also kinase proteins essential for neuronal development in lower organisms, such as unc-51 and MINK, were revealed by the screen.30, 31, 32, 33 Similarly, the identification of JAK1 and JAK2 kinases of the JAK/STAT pathway for neurite outgrowth (Table 1) is consistent with a previously described function of STAT3 activation, which is necessary to increase growth of dorsal root ganglion neurons and improve axonal regeneration in spinal cord after a conditioning injury.34
Nevertheless, the majority of the candidates, many of which have housekeeping functions such as mitochondrial DNA (mtDNA) synthesis and cell cycle-related kinases (Table 2), have not been described to influence neurite outgrowth. For example, in non-replicating cells such as neurons, where cytosolic deoxyribonucleotide triphosphate synthesis is downregulated, mtDNA synthesis depends solely on the mitochondrial salvage pathway enzymes, deoxyribonucleoside kinases. Intriguingly, RNAi downregulation of all four mammalian deoxyribonucleoside kinases, DCK, TK1, TK2 and DGUOK, promote neurite outgrowth. Mutations in DGUOK and TK2 have been found in patients with mtDNA depletion syndromes and 10–15% of these patients exhibit neuronal abnormalities.35 The identification of all deoxyribonucleoside kinases as negative regulators of neurite outgrowth suggests that mtDNA synthesis machinery negatively regulates neurite outgrowth and is essential for neuronal function.
Cell cycle exit represents the fundamental step to trigger differentiation of cells. It is therefore not surprising that downregulation of check point kinases, such as CHEK1, CDK11, CDK9 and CLK4, led to inhibition of neurite outgrowth (Table 1). These cannot be formally validated as direct modulators of axodendritic length, without testing their role in non-dividing cells. On the other hand, downregulation of a subset of cell cycle kinases, BUB1, AURKC, PLK4 and members of the NEK kinase family, promotes the outgrowth of neurites in our assay condition, suggesting that functions unrelated to cell cycle progression are modulated by these proteins. Indeed, recent convergence of data indicates a relationship between cilia, cell size and cell cycle progression.36 Based on our screen results, it is conceivable that signalling pathways regulating cytoskeletal rearrangement may be similar for both cell cycle progression and neurite outgrowth, and that the so-called cell cycle kinases may have a wider role.
Finally, our outgrowth screen also identified CKM and CKMT2, two major isozymes of creatine kinase, as negative regulators of neurite outgrowth. The importance of these kinases as negative regulators of neurite outgrowth is further confirmed by the dopaminergic neuronal culture regeneration model (Figure 5). Supplementation of the substrate of this kinase, creatine, has been shown to protect neurons against neurotoxins in vitro and was able to slow down the progression of a number of neurodegenerative disease states in experimental animal models.37, 38 Our finding further highlights the role of creatine kinase in neuroprotection and as a putative target for drugs aimed to promote neuronal regeneration and inhibit neuronal degeneration.
LPA-induced neurite retraction involves Rho GTPase signalling and ROCK1, which is a well-characterized mediator of neurite regression.25, 39 ROCK is crucial for mediating the growth inhibitory signal of myelin proteins in the CNS and its inhibition has been shown to increase axonal regeneration in vivo.40 In addition to ROCK1, a series of kinases previously unrelated to neurite degeneration was identified by the screen (Table 3). Among others, PI3K (via AKT and PKC) and cytoplasmic and receptor TK signalling (EGFR, FGFR, PDGFR and FLT1) were essential for LPA-induced neurite retraction. To validate both known and novel kinases revealed by the screen, we selected ROCK1 and a member of the PKC superfamily, PKN1. Haploinsufficiency of either rok, a Drosophila orthologue of human ROCK1, or PKN1 was able to suppress light-induced retinal degeneration in the fly model of ADRP.
Interestingly, 20 candidate kinases shared the property of negative modulators of neurite outgrowth as well as positive modulators of LPA-induced neurite retraction (Table 3). The integrity of the cytoskeleton is essential for the maintenance of axon stability, and axonal retraction requires intimate interaction between actin and microtubule cytoskeleton.41 It is likely that this subset of kinases is involved in the signalling pathways of the stabilization or destabilization of microtubules, actin or interactions between the two cytoskeletal systems.
The observation of subgroup clustering for all candidates in both screens suggests a different role for different kinase subgroups in neuronal development. For example, members of the AGC, OTHER and ATYPICAL subgroups were predominantly negative modulators of neurite outgrowth and retraction, whereas those of the TK, TKL and STE subgroups were predominantly positive modulators of neurite outgrowth (Figure 3). In addition, clustering of different kinase subgroups (Figure 3b and Supplementary Figure 4) suggests that complex networks involving apparently unrelated groups of kinases are required for neuronal differentiation, degeneration and regeneration. The study of Figure 3b and Supplementary Figure 4 also suggests that complex processes such as neurite length regulation are poorly described by classical, hierarchically arranged, vertical pathways.
This study is far from being an in-depth analysis of all kinases involved in the regulation of neurite length, but provides the first overall picture of the complex signalling network that involves different kinase families in neurite outgrowth and the opposite process of neurite retraction. It also identifies new putative cross-talks between previously unrelated signalling pathways. The mechanisms involved in degeneration of neuronal bodies and their processes, axons and dendrites, involve spatial and temporal signal segregation.4 The results of this screen are a template for further studies aimed to understand the molecular interaction leading to degeneration/outgrowth of neuronal processes and will help selection of appropriate targets for the design of new therapeutic strategies in neurodegenerative conditions. Protection of axonal and dendritic degeneration and stimulation of the regenerative capacity in damaged neurons might be the best option to improve clinical outcome in human neurodegenerative disorders.
Materials and Methods
siRNA library
A custom siRNA library targeting 750 human kinases with three-fold redundancy was obtained from Ambion Inc. The three siRNA duplexes for each target were individually arrayed in a 96-well format (Supplementary Figure 1). Using Fam-labelled siRNA-targeting ROCKII, greater than 92% transfection efficiency was observed (Supplementary Figure 2) and, on average, greater than 66% ‘knockdown’ for each mRNA target species was observed (Supplementary Figure 3).
High-throughput neurite outgrowth and retraction assays
SH-SY5Y cells were seeded on 96-well plates, at density of 10 000 cells per well, in non-differentiating medium (DMEM with 10% FCS plus 1%P/S) and cultured overnight at 37°C. 24 h post-seeding, non-differentiating medium was replaced with differentiating medium (Neurobasal medium (Invitrogen) supplemented with B27 supplement (Invitrogen), 1%v/v Glutamine and 1 mM db-cAMP (Sigma)) and the cells were transfected with 50 nM siRNA using Lipofectamine 2000 reagent (Invitrogen). Forty-eight hours post-transfection, the cells were fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature (RT) followed by treatment for 5 min with 0.1% Triton-X-100 and 50% normal goat serum (NGS) in PBS for 1 h at RT. Neuronal-specific anti-βIII tubulin antibody (Promega) was used to stain the neurite processes at a dilution of 1 in 5000 in 50% NGS at RT for 3–4 h or 4°C overnight with low constant agitation. Bound antibody and nucleus were visualized using Alexa 546-conjugated secondary antibodies (Molecular Probe, 1 in 500) and Hoechst 33342 (Molecular Probe, 1 in 1000), respectively. Retraction assay was identical to the outgrowth assay as mentioned above, except for the addition of 10 μM LPA (Sigma) for an hour before fixing the cells.
Automated images analysis
Cells were imaged using a Cellomics Kinetic Scan Reader high content microscope system and analysed using neurite outgrowth and extended neurite outgrowth BioApplications for the outgrowth and retraction assays, respectively. Twenty images per well per sample were taken at × 10 magnification in a fully automated and blind manner. The total number of cell counts and the average neurite length per neuron and average neurite length per neurite for duplicated experiments were determined for neurite outgrowth and retraction assays, respectively.
CGNs culture and siRNA transfection
CGNs were prepared from 7-day-old Wistar rat pups and cultured in BME medium (Invitrogen) supplemented with 10% FCS, 25 mM KCl, 1% Glutamax and 1% P/S. Delivery of siRNA into CGNs was performed using the Amaxa nucleofection system (Amaxa GmbH). Optimization was performed with the Amaxa RNAi test kit using pmaxGFPTM plasmid and siRNA directed against pmaxGFP. Following optimization, 6 million cells were co-nucleofected with pmaxGFP and either scrambled siRNA control or gene-specific siRNAs (Qiagen) at a final concentration of 50 nM. Nucleofected cells were plated on poly-L-lysine coated six-well plates and 48 h post-nucleofection cells were fixed with 3% paraformaldehyde. A minimum of 30 images of GFP-positive CGNs was acquired randomly from each well at × 20 magnification using a Zeiss Axiovert 40 fluorescence microscope. The length of all neurite processes of at least 85 neurons per sample were traced using NeuronJ 1.01 imaging software (Erik Meijering) by two independent researchers. The total neurite length/neuron (μm) was calculated and plotted as scatter plot for all samples traced. The data were analysed by using Student's t-test compared to scrambled siRNA control and differences were considered to be significant from P<0.05.
In vitro regeneration assay and quantitative analysis of axonal regeneration
Primary midbrain cultures were prepared from embryonic rats on day 14 of gestation (E14) as previously described26 and maintained in DMEM-F12 (Invitrogen) supplemented with 2.5 mg/ml BSA (35%), 0.9% D-(+)-glucose solution (45%), 2 mM L-glutamine (PAA Laboratories, Pasching, Austria), 5 μg/ml insulin, 1 : 100 N1 medium supplement and 1 : 100 PSN antibiotic mixture (Invitrogen). Half of the culture medium was exchanged at DIV 1 and subsequently every second day. Delivery of siRNA into CGNs was performed using the Amaxa nucleofection system (Amaxa GmbH). Two million cells were nucleoporated with a pool of two gene-specific siRNAs (Qiagen) at a final concentration of 200 nM. Nucleoporated cells were plated on poly-L-ornithine/laminin-coated coverslips in 24-well plates at a density of 5 × 105 cells per well. On DIV 3, a close neurital network was established in culture. A scratch was performed on the surface of the glass coverslip using a 2 mm thick rubber scraper resulting in a lesion on which axonal regeneration could be studied. Each coverslip was microscopically examined to ensure completeness of the scratch. To assess overall neurite outgrowth into the scratch, eight live images per condition were acquired at a random location along the scratch on DIV 6 using an inverted microscope (Axiovert 200 M; Carl Zeiss, Göttingen, Germany). To specifically quantify regeneration of dopaminergic neurons into the scratch, the culture was fixed with 4% paraformaldehyde for 10 min at RT followed by permeabilizing with 100% acetone for 10 min at −20°C and blocking with 10% NGS in PBS for 10 min at RT. Anti-TH antibody (Advanced ImmunoChemical, Long Beach, USA) was used to stain for TH at a dilution of 1 : 250 in 10% NGS overnight at 4°C. Bound antibody and nucleus were visualized using Cy2-conjugated goat anti-rabbit secondary antibody (Abcam, Cambridge, UK) and 4′,6-diamidino-2-phenylindole (DAPI), respectively. Eight images per condition were captured. Quantification of the length of regenerating neurites was performed with the NeuronJ plug-in of the ImageJ software. In each image, the 10 longest processes were measured and averaged. For statistical analysis, all cell culture experiments were repeated at least twice.
Drosophila retinal degeneration assay
The following genotypes were used in this study: ninaEG69D and ninaERH27 were used as models for the class III ADRP,27, 28 PKNrG232/Cyo, PKNk11209/Cyo, rok[1]/FM7i, rok[2]/FM7i and rok[f04065]/FM7i were from Bloomington stock center. Fly stocks were maintained on standard cornmeal agar media at 25°C in 12 h daylight and 12 h darkness. For light-induced retinal degeneration assay, 20–30 flies with the relevant genotypes were selected and cultured in glass vials at 25°C in permanent light (around 2000 lux) or darkness for 4 weeks. The vials were changed frequently to avoid mixing of the flies with eventual progeny. Tangential plastic sections were performed as previously described,42 and toluidine blue was used as a dye to increase the contrast.
Abbreviations
- AD:
-
Alzheimer's disease
- CNS:
-
central nervous system
- CGN:
-
cerebellar granule neurons
- DIV:
-
days in vitro
- db-cAMP:
-
dibutyryl-cyclic AMP
- EGFP:
-
enhanced green fluorescent protein
- GFP:
-
green fluorescent protein
- LPA:
-
lysophosphatidic acid
- mtDNA:
-
mitochondrial DNA
- PKA:
-
protein kinase A
- PKG:
-
protein kinase G
- PKC:
-
protein kinase C
- RNAi:
-
RNA interference
- siRNA:
-
small interfering RNA
- TK/TKL:
-
tyrosine kinase/tyrosine kinase-like
References
Yuan J, Lipinski M, Degterev A . Diversity in the mechanisms of neuronal cell death. Neuron 2003; 40: 401–413.
Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC . Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci 2000; 20: 1333–1341.
Raff MC, Whitmore AV, Finn JT . Axonal self-destruction and neurodegeneration. Science 2002; 296: 868–871.
Berliocchi L, Fava E, Leist M, Horvat V, Dinsdale D, Read D et al. Botulinum neurotoxin C initiates two different programs for neurite degeneration and neuronal apoptosis. J Cell Biol 2005; 168: 607–618.
Dent EW, Gertler FB . Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003; 40: 209–227.
Yoshimura T, Arimura N, Kaibuchi K . Signaling networks in neuronal polarization. J Neurosci 2006; 26: 10626–10630.
Dancey J, Sausville EA . Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2003; 2: 296–313.
Melnikova I, Golden J . Targeting protein kinases. Nat Rev Drug Discov 2004; 3: 993–994.
Smith PD, O'Hare MJ, Park DS . Emerging pathogenic role for cyclin dependent kinases in neurodegeneration. Cell Cycle 2004; 3: 289–291.
Angelo M, Plattner F, Giese KP . Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem 2006; 99: 353–370.
Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J . Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 2001; 20: 27–39.
Hatano Y, Li Y, Sato K, Asakawa S, Yamamura Y, Tomiyama H et al. Novel PINK1 mutations in early-onset parkinsonism. Ann Neurol 2004; 56: 424–427.
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 2004; 304: 1158–1160.
Klein C, Schlossmacher MG . The genetics of Parkinson disease: implications for neurological care. Nat Clin Pract Neurol 2006; 2: 136–146.
Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 2004; 44: 595–600.
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–607.
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006; 441: 1157–1161.
Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem 2005; 280: 34025–34032.
Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 2006; 15: 223–232.
West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 2005; 102: 16842–16847.
Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA 2005; 102: 18676–18681.
Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA . Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 2006; 9: 1231–1233.
Branchi I, Bichler Z, Minghetti L, Delabar JM, Malchiodi-Albedi F, Gonzalez MC et al. Transgenic mouse in vivo library of human Down syndrome critical region 1: association between DYRK1A overexpression, brain development abnormalities, and cell cycle protein alteration. J Neuropathol Exp Neurol 2004; 63: 429–440.
Lee G, Tanaka M, Park K, Lee SS, Kim YM, Junn E et al. Casein kinase II-mediated phosphorylation regulates alpha-synuclein/synphilin-1 interaction and inclusion body formation. J Biol Chem 2004; 279: 6834–6839.
Katoh H, Aoki J, Ichikawa A, Negishi M . p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem 1998; 273: 2489–2492.
Lingor P, Unsicker K, Krieglstein K . GDNF and NT-4 protect midbrain dopaminergic neurons from toxic damage by iron and nitric oxide. Exp Neurol 2000; 163: 55–62.
Colley NJ, Cassill JA, Baker EK, Zuker CS . Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci USA 1995; 92: 3070–3074.
Kurada P, O'Tousa JE . Retinal degeneration caused by dominant rhodopsin mutations in Drosophila. Neuron 1995; 14: 571–579.
Davidson FF, Steller H . Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 1998; 391: 587–591.
Poinat P, De Arcangelis A, Sookhareea S, Zhu X, Hedgecock EM, Labouesse M et al. A conserved interaction between beta1 integrin/PAT-3 and Nck-interacting kinase/MIG-15 that mediates commissural axon navigation in C elegans. Curr Biol 2002; 12: 622–631.
Tomoda T, Kim JH, Zhan C, Hatten ME . Role of Unc51.1 and its binding partners in CNS axon outgrowth. Genes Dev 2004; 18: 541–558.
Minichiello L, Casagranda F, Tatche RS, Stucky CL, Postigo A, Lewin GR et al. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron 1998; 21: 335–345.
Murai KK, Pasquale EB . New exchanges in eph-dependent growth cone dynamics. Neuron 2005; 46: 161–163.
Qiu J, Cafferty WB, McMahon SB, Thompson SW . Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 2005; 25: 1645–1653.
Spinazzola A, Zeviani M . Disorders of nuclear-mitochondrial intergenomic signaling. Gene 2005; 354: 162–168.
Quarmby LM, Parker JD . Cilia and the cell cycle? J Cell Biol 2005; 169: 707–710.
Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci 2000; 20: 4389–4397.
Brewer GJ, Wallimann TW . Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 2000; 74: 1968–1978.
Fukushima N . LPA in neural cell development. J Cell Biochem 2004; 92: 993–1003.
Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bahr M et al. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem 2007; 103: 181–189.
Ahmad FJ, Hughey J, Wittmann T, Hyman A, Greaser M, Baas PW . Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat Cell Biol 2000; 2: 276–280.
Tomlinson A . The cellular dynamics of pattern formation in the eye of Drosophila. J Embryol Exp Morphol 1985; 89: 313–331.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S . The protein kinase complement of the human genome. Science 2002; 298: 1912–1934.
Acknowledgements
We thank Veronique Planchamp and Anja Krauss for assistance in dopaminergic regeneration assay, Roger Snowdon for assistance in FACS analysis and high throughput screening, Maria Guerra Martin for preparation of CGN cultures, Pedro Domingos (HHMI, The Rockefeller University) and Bloomington Stock Center for fly strains, the Kyriacou lab members for technical assistance in fly work, David Dinsdale, Judy McWilliams and Tim Smith for assistance with TEM, Paul Glynn for critical paper review and Shu Dong Zhang for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Loh, S., Francescut, L., Lingor, P. et al. Identification of new kinase clusters required for neurite outgrowth and retraction by a loss-of-function RNA interference screen. Cell Death Differ 15, 283–298 (2008). https://doi.org/10.1038/sj.cdd.4402258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402258
Keywords
This article is cited by
-
AAV-mediated inhibition of ULK1 promotes axonal regeneration in the central nervous system in vitro and in vivo
Cell Death & Disease (2021)
-
Inhibition of the autophagic protein ULK1 attenuates axonal degeneration in vitro and in vivo, enhances translation, and modulates splicing
Cell Death & Differentiation (2020)
-
High MAST2 mRNA expression and its role in diagnosis and prognosis of liver cancer
Scientific Reports (2019)
-
Transcription factor activating protein 4 is synthetically lethal and a master regulator of MYCN-amplified neuroblastoma
Oncogene (2018)
-
A kinome-targeted RNAi-based screen links FGF signaling to H2AX phosphorylation in response to radiation
Cellular and Molecular Life Sciences (2015)