Abstract
Idiopathic-dilated cardiomyopathy (IDC) is a common primary myocardial disease of unknown etiology associated with apoptosis, cardiac dilatation, progressive heart failure and increased mortality. An elevation of the transcription factor activator protein 2α (AP-2α) is involved in vertebrate embryonic development and oncogenesis. Here, we show that AP-2α protein is expressed in the human heart and increased in human failing myocardium with IDC. Adenovirus-mediated overexpression of human AP-2α triggered apoptosis and increased mRNA levels of Bcl-2 family members Bax and Bcl-x in rat cardiomyocytes. Immunohistological analysis of human myocardium revealed an increased percentage of AP-2α-positive nuclei in IDC and, interestingly, a colocalization of AP-2α-positive but not -negative cells with a caspase-cleaved fragment of poly(ADP-ribose)polymerase. We suggest AP-2α as a novel cardiac regulator implicated in the activation of apoptosis in IDC.
Similar content being viewed by others
Introduction
Congestive heart failure is the end stage of various cardiac diseases defined by the heart's inability to cover adequately the body's blood demand.1 It is associated with a poor prognosis and is the leading cause of cardiovascular morbidity and mortality in Western countries.2 The idiopathic-dilated cardiomyopathy (IDC) is a common primary myocardial disease of unknown etiology representing a major cause for heart failure and heart transplantations.3 It is characterized by massive ventricular dilatation, impaired cardiac contraction and relaxation,4 and apoptosis as observed in human heart failure due to IDC and other forms of heart failure.5 Expressional changes of cardiac regulatory proteins can in part explain functional alterations of the failing heart and are hypothesized to play a key role in the pathophysiology of heart failure.6 However, little is known about molecular mechanisms of gene regulation during initiation and progression of heart failure and leading to the activation of apoptosis in failing heart.
The activator protein 2 (AP-2) family of transcription factors consists of the structurally related DNA-binding proteins AP-2α, AP-2β, AP-2γ and AP-2δ.7,8,9,10,11 AP-2 proteins regulate the transcription of a variety of genes (e.g. growth factors and growth factor receptors) through the AP-2 consensus element GCCNNNGGC,12 and AP-2 activity can be induced by different stimuli, for example, by phorbol esters, cAMP or retinoic acid.13,14,15,16 The expression of AP-2α is associated with the embryonic differentiation of neuroectodermal, urogenital and ectodermal tissues;17,18 mice lacking the AP-2α gene are not viable and show severe defects of craniofacial development.19,20 An increase of AP-2α in various human breast cancer cell lines is associated with the induction of the proto-oncogene c-erb-B2 and with a highly aggressive tumor phenotype,21,22 suggesting a role of AP-2α in cell growth and oncogenesis. AP-2α has also been suggested as a tumor suppressor gene in melanoma cells.23
Various studies supported the hypothesis that chronic β-adrenergic stimulation of the cAMP-dependent signaling pathway by elevated plasma catecholamines contributes to the altered expression of functionally relevant cardiac genes in human failing heart.24 β-adrenoceptor antagonists improve cardiac function and prognosis in patients suffering from heart failure due to IDC,25 and restore expressional alterations of myocardial regulatory proteins.26,27 Vice versa, β-adrenoceptor agonists increase the mortality in heart failure patients 28 and lead to morphological, functional and expressional changes in rats similar to human heart failure.29 Thus, chronic β-adrenergic stimulation of the cAMP-dependent signaling pathway by elevated plasma catecholamines is implicated in deleterious expressional changes in the failing heart.
Here, we studied the expression of the cAMP-dependent transcription factor AP-2α in human failing hearts as well as effects of adenovirus-mediated gene transfer of AP-2α in primary rat cardiac myocytes. AP-2α protein was increased in ventricular specimens from failing hearts with IDC. Adenovirus-mediated expression of AP-2α triggered apoptosis in cardiac myocytes and increased mRNA levels encoding Bax and Bcl-x. AP-2α-mediated apoptosis was not inhibited by the p53 inhibitor pifithrin-α. We suggest AP-2α as a novel mediator of apoptosis contributing to the pathophysiology of human heart failure.
Results
AP-2α is expressed in the human heart and upregulated in IDC
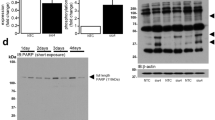
AP-2α protein was immunologically detected at 45 kDa in the right ventricular homogenate of a human failing heart with IDC (Figure 1a). The immunoreactivity of this protein band was inhibited by preincubation of the antibody with the immunizing peptide consisting of the 18 C-terminal amino acids of the activating splice variant AP-2αA, commonly named AP-2α;13 the signal was not inhibited by a nonrelated peptide derived from transcription factor SP-1, indicating signal specificity. Additional immunreactive bands of about 65 and 80 kDa that were also blocked by preadsorption with the immunizing peptide have been previously described in human mammary tumor cell lines and are not yet identified.21 AP-2α protein levels were not different in both ventricles of failing human hearts with IDC (PhosphorImager units; mean±S.E.M: right ventricle 311±45, n=9; left ventricle 341±100, n=10). Since elevation of AP-2α represents a major mechanism of transcriptional activation through the AP-2 element,13,14,15,16 we compared AP-2α protein levels in right ventricular tissue samples from six human nonfailing (NF) donor hearts and from seven failing hearts with IDC. Compared to the NF controls, the protein levels of AP-2α were increased to 220% in IDC (Figure 1b and c; AP-2α: IDC, 338±54*; NF, 152±23, *P<0.05). The 65 and 80 kDa bands recognized by the AP-2α-specific antibody were also increased to more than 200% (data not shown); however, the relevance of this finding is not clear. Protein levels of calsequestrin (CSQ), a calcium-storing protein of the sarcoplasmic reticulum, were determined in the same samples as negative control. CSQ levels were not different in both groups indicating equal loading of the blots (Figure 1b and c; CSQ: IDC, 117±14; NF, 101±16).
Expression of AP-2α in the human heart. (a) Immunological detection of AP-2α. AP-2α protein was detected at 45 kDa in a human cardiac ventricular homogenate (IDC; lane 1) using a polyclonal peptide antibody specific for AP-2α and 125I-labeled protein A for visualization. The signal was blocked by preadsorption with the immunizing peptide (lane 2) but not with a nonrelated peptide derived from the transcription factor SP-1 (lane 3). (b) Autoradiographies of immunoblots for AP-2α and CSQ, a sarcolemmal protein used for standardization, with homogenates from an NF and a failing human cardiac ventricle (IDC). Signals were visualized using 125I-labeled protein A. (c) Bar graph showing the quantification of AP-2α and CSQ in ventricular homogenates from seven failing (IDC) and six NF hearts. Signals were obtained by quantification of the radioactivity of 125I-labeled protein A. In IDC, AP-2α protein was increased to more than 200% compared to NF, whereas CSQ was unchanged in both groups. *P<0.05 versus NF
Adenovirus-mediated overexpression of AP-2α triggers apoptosis in cardiac myocytes
In order to evaluate a specific cardiac role of AP-2α, we generated a recombinant adenovirus for the overexpression of human AP-2α in neonatal rat cardiomyocytes (Ad-AP-2α; Figure 2a). Cardiomyocytes were infected with Ad-AP-2α at various concentrations and cells were harvested 1, 2 and 3 days after infection (Figure 2b and c) before the expression of AP-2α was determined on immunoblots. AP-2α protein was increased in cardiomyocytes after infection with Ad-AP-2α but not with Ad-Ctr (Figure 2b). This increase was dependent on multiplicity of infection (MOI, Figure 2b) and on time post infection with a maximal expression after 24 h (Figure 2c). AP-2α-expressing cardiomyocytes showed cell shrinkage and nuclear condensation that are morphological signs of apoptotic death30 (Figure 3a); the degree of these alterations was dependent on the MOI applied. More than 90% of Ad-AP-2α-infected cells were dead – rounded up, shrinked and detached from the culture dish – 3 (MOI=10) to 5 days (MOI=3.5) post infection (not shown). These alterations were not observed in Ad-Ctr-infected cells (Figure 3b) using the same virus concentrations as controlled by green fluorescent protein (GFP) expression (not shown) and by immunological determination of the adenoviral protein E2a (Figure 3c). DNA of infected cardiomyocytes was isolated and separated by agarose-gel electrophoresis to study DNA fragmentation as a typical phenomenon of apoptosis.30,31 Apoptosis-specific DNA fragments with sizes of multiples of 180 bp were only observed in Ad-AP-2α-infected cells but not after infection with Ad-Ctr (Figure 4a). Staining with the nucleic acid dye 4′,6′-diamidino-2-phenylindole (DAPI) revealed nuclear alterations including condensation and margination of chromatin in Ad-AP-2α-infected cardiomyocytes but not after infection with Ad-Ctr (Figure 4b). Since caspases are central regulators of apoptosis,32 the proteolytic activity of caspases was studied in infected cardiac myocytes using the caspase substrate DEVD in a luminometric assay (Figure 4c). Proteolytic activity of caspases was significantly increased 3 days after infection with Ad-AP-2α reaching a plateau on day 4. Activation of caspases by AP-2α was apparently independent from transcription factor p53, a central regulator of apoptosis,33 since a similar extent of caspase activation was observed in the presence of pifithrin-α, an inhibitor interfering with p53-mediated transactivation.34 In order to identify apoptosis-related target genes of AP-2α in cardiomyocytes, we studied the mRNA levels of Fas (CD95), Bcl-x, Fas ligand (FasL), caspase-1, caspase-2, caspase-3, Bax and Bcl-2 in Ad-AP-2α- and Ad-Ctr-infected cardiomyocytes using RNase protection assays (Figure 5). While mRNA levels of Fas, caspase-2 and caspase-3 and of L32, a ribosomal housekeeping gene, were not different in both groups, mRNA levels of Bcl-x and of Bax were increased to 153 and 137% in Ad-AP-2α infected cardiomyocytes, respectively; mRNA levels for FasL, caspase-1 and Bcl-2 were below the detection limit.
Adenovirus-mediated overexpression of AP-2α in rat neonatal cardiomyocytes. (a) Map showing the structure of Ad-AP-2α. Ad-AP-2α was deleted in the E1 and E3 regions and contained two expression cassettes for the GFP and AP-2α, both under the control of the cytomegalovirus early gene promoter. (b) Immunological detection of AP-2α on a Western blot of rat neonatal cardiomyocytes 2 days after infection with Ad-AP-2α at different MOIs. AP-2α was detected at 45 kDa, and the signal was increased with the MOI applied. There was no elevation of AP-2α protein detectable after infection with Ad-Ctr. (c) Immunological detection of AP-2α on a Western blot of rat neonatal cardiomyocytes 0, 1, 2 and 3 days after infection with Ad-AP-2α at an MOI of 10. The overexpression of AP-2α was maximal 1 day post infection
Light-microscopic photographs of rat neonatal cardiomyocytes after infection with Ad-AP-2α. (a) At 2 days post infection with Ad-AP-2α cardiomyocytes showed morphological alterations such as cell shrinkage and nuclear condensation depending on the MOI applied. (b) Cardiomyocytes were infected with Ad-AP-2α or Ad-Ctr at an MOI of 10, respectively. The morphological alterations were only observed in Ad-AP-2α-infected cells but not after infection with Ad-Ctr applied at the same MOI as confirmed by immunological detection of the adenoviral protein E2a (c)
Determination of apoptosis in Ad-AP-2α-infected cardiomyocytes. (a) Fragmentation of DNA (‘DNA-laddering’) into fragments of multiples of 180 bp in size was observed in Ad-AP-2α-infected cardiomyocytes but not in Ad-Ctr-infected cells (MOI=10). The size of DNA fragments is indicated in kilobases on the left side. (b) Morphology of DAPI-stained nuclei: apoptotic nuclei with chromatin condensation and margination were only detected after infection with Ad-AP-2α, but not after infection with Ad-Ctr (MOI=10). (c) Proteolytic activity of caspases in cardiomyocytes infected with Ad-Ctr (○; n=3–4) or Ad-AP-2α in the absence (•; n=4) or presence of the p53 inhibitor pifithrin-α (▪; n=4; 10 μM) at an MOI of 3.5; RLU=relative light units produced by DEVD-conjugated luciferin. Note that caspase activation 3–5 days after infection with Ad-AP-2α (P<0.05 versus Ad-Ctr) was not affected by pifithrin-α
Expression of apoptosis-related genes in Ad-AP-2α-infected cardiomyocytes. (a) Representative autoradiography of RNase-protected mRNAs encoding Fas, Bcl-x, caspase-3 (Csp3), caspase-2 (Csp2), Bax and the housekeeping genes L32 and GAPDH from rat cardiomyocytes 2 days after infection with Ad-AP-2α (A) and Ad-Ctr (C) at an MOI of 3.5. Note increased mRNA levels for Bcl-x and Bax in Ad-AP-2α-infected cardiomyocytes. (b) Statistical analysis of mRNA levels encoding apoptosis-related genes from several independent experiments in cardiomyocytes infected with Ad-Ctr (open bars, n=8) or Ad-AP-2α (filled bars, n=12). *P<0.05 versus infection with Ad-Ctr
Colocalization of caspase-cleaved poly(ADP-ribose)polymerase (PARP) with AP-2α in the human myocardium
In order to study a possible role of AP-2α in the human heart, we performed an immunohistological analysis of right ventricular tissue specimens from NF donor hearts in comparison to failing hearts with IDC using the AP-2α-specific antibody (green fluorescence; Figure 6). AP-2α was localized in the nuclei of myocytes as obvious from counterstaining with the nuclear dye DAPI (blue fluorescence), and the percentage of AP-2α-positive nuclei was significantly higher in failing hearts as compared to NF controls (Table 1). Tissue sections were costained with an antibody recognizing caspase-cleaved PARP (red fluorescence) to study the possible role of AP-2α-positive and -negative cells in cardiac apoptosis. The fraction of cleaved PARP-positive cells was considerably elevated in failing hearts as compared to NF hearts, which revealed only a faint staining for caspase cleavage. While cleaved PARP was not observed in AP-2α-negative cells, a substantial fraction of AP-2α-positive cells showed colocalization with cleaved PARP, suggesting that AP-2α is implicated in proapoptotic caspase activation in human heart failure due to IDC.
Immunohistological localization of AP-2α and cleaved caspase-3 in human failing and NF ventricular myocardium. Tissue sections from a failing human heart with IDC (left column) and an NF human control heart (NF, right column). (a, b) Staining with the nuclear dye DAPI (blue). (c, d) Immunological staining of caspase-cleaved PARP (red). Arrows indicate positively stained cells. Smaller red spots were assigned as nonspecific signals, putatively due to staining of lipofuscin granula, by performing negative control staining reactions lacking the first antibody (not shown). (e, f) Immunological staining of AP-2α (green). (g, h) Overlay. Stars indicate AP-2α-positive cells, arrows indicate colocalization of cleaved PARP and AP-2α
Discussion
This is the first report of a functional role of AP-2α in myocardial apoptosis showing (i) that AP-2α is upregulated in human failing ventricles with IDC colocalizing with caspase-cleaved PARP and (ii) that adenovirus-mediated overexpression of AP-2α triggers apoptosis in cardiac myocytes. The activating isoform AP-2αA (commonly named AP-2α) was identified in human ventricular tissue using an antibody raised against the C-terminus of AP-2αA, a region which is not homologous to AP-2β, AP-2γ, AP-2δ or the inhibitory splice variant AP-2αB.13 This result agrees with results from DNaseI footprint assays where an AP-2 consensus element-specific binding activity was observed in adult rat cardiac protein extracts.35 Previously, the expression of AP-2α was assigned to neural crest-derived cell types in mice as assessed by in situ hybridization;17,18 only a weak and transient expression of AP-2α mRNA was found in mouse hearts during early embryonic development and there was no AP-2α mRNA detectable in adult mouse hearts. The difference in our data may be explained by species differences and by our observation of a strong expression of AP-2α only in failing human hearts with IDC.
Elevation of AP-2α represents a major mechanism for transcriptional activation through the AP-2 element.13,14,15,16 More recently, the modulation of AP-2α activity by phosphorylation has been reported, but the functional role of this mechanism is not clear.36 Elevation of AP-2α was observed after stimulation of both cAMP- or retinoic acid-dependent signaling pathways 13,36,14 and can be explained by a positive autoregulation through AP-2 elements in the AP-2α gene promoter 16,37 and a regulation by the transcriptional repressor AP-2rep.16 Essential cellular processes such as differentiation and growth control are regulated by AP-2α,10,11,12,13,15,38 and AP-2α is implicated in the pathophysiology of human breast cancer.21,22 Therefore, it is conceivable that the upregulation of AP-2α in failing human heart, possibly as a consequence of chronic β-adrenergic stimulation of the cAMP-dependent signaling pathway by elevated plasma catecholamines, contributes to the regulation of deleterious expressional changes during initiation or progression of human heart failure.
Overexpression of human AP-2α led to the activation of apoptosis in cardiomyocytes as assessed by different criteria including cellular and nuclear morphology, fragmentation of genomic DNA (DNA laddering) and elevation of the proteolytic activity of caspases. Apoptosis has been described in heart failure due to IDC and other cardiac diseases,5 and beneficial effects of a long-term therapy with angiotensin-converting enzyme inhibitors as well as carvedilol, an antagonist at β- and α-adrenergic receptors, may involve the inhibition of cardiac apoptosis.39,40,41 Up to now, the molecular pathways leading to the activation of the apoptotic signaling cascade, in particular, in the heart are not understood in detail. AP-2α has been shown to inhibit the activation of apoptosis mediated by the proto-oncogene c-myc,42 and recently, AP-2α has been implicated in the antiapoptotic effects of the retinoblastoma gene product and in the induction of Bcl-2 in epithelial but not in NIH 3T3 mesenchymal cells.43 AP-2α has also been recently reported to interact directly with p53 and to augment p53 transcriptional activation.33 This finding would be in line with our data that Bax, a classical proapoptotic target gene of p53, is increased after overexpression of AP-2α. However, our observation that pifithrin-α, an inhibitor of p53 transcriptional activation, did inhibit neither AP-2α-induced caspase activation nor apoptosis (data not shown) would argue against an essential role of p53. Nevertheless, given the complex and multifaceted nature of p53 actions, a contribution of p53 in AP-2α-triggered apoptosis can formally not be excluded. Interestingly, the upregulation of Bcl-2 family proteins including Bax and Bcl-x has been described in human end-stage failing hearts,44 which is similar to our data showing the upregulation of Bax and Bcl-x mRNAs in Ad-AP-2α-infected cardiomyocytes. Therefore, our results suggest a dual regulation of apoptosis by AP-2α with both activation and inhibition of apoptotic signaling pathways, possibly depending on the cell type. Our hypothesis of a functional role of AP-2α in myocardial apoptosis is further supported by immunohistological data that only AP-2α-positive cells, but not AP-2α-negative cells, displayed cleavage of PARP, suggesting that AP-2α is required for caspase-3-dependent apoptosis in the heart. However, the majority of AP-2α-positive cells was not apoptotic, indicating that AP-2α alone may not be sufficient for the activation of caspases or that a certain duration or threshold of AP-2α expression is required for caspase activation. The percentage of AP-2α- and cleaved PARP-positive nuclei was elevated in failing myocardium (IDC) as compared to NF controls. This result agrees well with the elevation of AP-2α protein in failing myocardium observed in immunoblots and with published data describing increased activation of apoptosis in human heart failure.5 The percentage of cleaved PARP-positive cells in failing myocardium (about 13%) is considerably higher as reported values obtained by TUNEL assays and other methods based on the detection of fragmented DNA.5 The comparative evaluation of different methods to monitor apoptosis was addressed previously,45 and more than 20% cleaved PARP-positive cells but less than 0.5% TUNEL-positive cells were detected in hepatitis C-virus-infected liver tissue using the same antibody. This supported the view that the activation of the caspase cascade is not an irreversible process, indicating cleaved PARP as a marker of early events during the activation of apoptosis.
Altogether, we suggest AP-2α as a central cardiac regulator protein contributing to the activation of caspases in human heart failure and possibly representing a target for novel therapeutic approaches. The finding that human hearts with IDC show an increase of AP-2α expression colocalizing with cleaved PARP together with the observation that adenoviral overexpression of AP-2α triggers the apoptotic cascade in cultured cardiomyocytes argues for a pathogenic role of AP-2α in cardiac failure. However, future studies on genetic mouse models with gain or loss of AP-2α function are required to further elucidate the functional role of AP-2α in this process.
Materials and Methods
Human myocardial tissue
Right ventricular tissue specimens were from seven patients with IDC undergoing orthotopic heart transplantation. Patients were clinically assigned to New York Heart Association class IV before transplantation. Ejection fraction and cardiac index were decreased corresponding with the clinical diagnosis and ranged from 15 to 40% and 1.3 to 2.5 l/min/m2, respectively. Regional distribution of AP-2α was studied in left and right ventricular tissue specimens from 10 explanted human failing hearts with IDC. Patients received standard medical therapy including digitalis, diuretics, inhibitors of angiotensin converting enzyme and nitrates, but no β-adrenoceptor antagonists. The study was approved by the local ethic committee and patients gave written informed consent. Right ventricular NF ventricular samples were from six transplant donors, which were brain-dead as a result of traumatic injury. Transplant donors routinely received low-dose dopamine to maintain renal perfusion. Their hearts were not transplanted for medical reasons and ventricular function was normal as determined by echocardiography. Tissue samples were frozen in liquid N2 and stored at −80°C for further analysis.
Preparation and infection of rat neonatal cardiac myocytes
Primary rat neonatal cardiomyocytes were prepared and maintained as described.46 Cells were infected with adenovirus in serum-free medium for 6 h at 37°C.
Construction of recombinant adenoviruses
A recombinant replication-deficient adenovirus type 5 for the overexpression of AP-2α (Ad-AP-2α) was generated by recombination of a shuttle plasmid (pAdTrack-CMV) containing the coding sequence of human AP-2α under the control of the cytomegalovirus early gene promoter and an E1-/E3-deleted adenoviral backbone plasmid (pAdEasy-1) in Escherichia coli (strain BJ5183).47 Subsequent transfection of recombinant virus DNA in HEK-293 cells was performed as described 47 and resulted in the production of infectious particles. The full-length cDNA of human AP-2α was a kind gift of Dr. R Buettner (Bonn, Germany) and was inserted in pAdTrack-CMV using NotI restriction sites. Recombinant Ad-AP-2α DNA (Figure 2a) was tested by Southern blotting after digestion with different restriction enzymes and hybridization with probes specific for the AP-2α insertion or the adenoviral backbone (data not shown). A second expression cassette for the GFP allowed the microscopical identification of infected cells and the determination of virus titers as GFP-expressing units/ml. A second virus, Ad-Ctr, was identical to Ad-AP-2α but lacking the insertion of the AP-2α coding sequence and served as a control. Virus stocks were prepared as described.47
Protein analysis
Preparation of cardiac homogenates, electrophoresis on 10% polyacrylamide gels and Western blotting onto nitrocellulose membranes were described elsewhere.48 Immunological detection was performed using polyclonal antibodies against AP-2αA (1 : 500; Santa Cruz, CA, USA) and CSQ49 (1 : 2000; kindly provided by Dr. LR Jones, Indianapolis, USA). CSQ, a sarcoplasmic Ca2+-storage protein, was reported to be unchanged in human heart failure 50 and was therefore used for standardization. Cardiac protein (70 μg) were loaded per lane for quantification, which was within the linear range of determination (data not shown). Cardiomyocytes were harvested and boiled for 15 min in 200 μl denaturation buffer 51 per 6-cm dish. The monoclonal antibody against E2a was described previously.52 Signals were visualized and quantified with 125I-labeled protein A and the PhosphorImager system (human specimens) or with alkaline phosphatase-coupled anti-rabbit IgG and color reagents according to standard protocols.
Analysis of nuclear morphology and DNA fragmentation
Infected cardiomyocytes were fixed and stained with the nucleic acid stain DAPI according to standard protocols. Fragmentation of DNA was studied as described.30 In brief, cardiomyocytes were lysed for 20 min at 4°C in a buffer containing 5 mM Tris-HCl, pH 7.4, 20 mM EDTA and 0.5% Triton X-100. The lysates were centrifuged at 20 000 × g for 15 min before supernatants were extracted with phenol–chloroform and nucleic acids precipitated in ethanol. The nucleic acids were electrophoresed on a 1.5% agarose gel. After this, the agarose gel was incubated for 3 h at 37°C with 20 μg/ml RNase A (Sigma, St. Louis, MO, USA) before it was stained with ethidium bromide and analyzed under UV light.
Fluorogenic substrate assay for caspases
Cardiomyocytes were kept in the presence or absence of 10 μM pifithrin-α (Alexis, San Diego, CA, USA), an inhibitor of p53 transcriptional activation.34 As a positive control, γ-irradiated MCF-7 cells were treated with 10 μM pifithrin-α, which completely abolished p53-mediated induction of p21WAF expression (data not shown). Fresh solution of pifithrin-α was applied daily with media change. Cytosolic extracts were prepared by lysing cells in a buffer containing 0.5% NP-40, 20 mM HEPES pH 7.4, 84 mM KCl, 10 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1 mM DTT, 5 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin and 1 mM PMSF. Caspase activities were determined in a luminometric assay using a luciferin-conjugated DEVD substrate (caspase-glo™ 3/7-assay, Promega, Madison, WI, USA) according to the manufacturer's specifications.
RNAse protection assay
Total RNA was extracted from adenovirus-infected cardiomyocytes using the RNeasy™ Midi Kit (Qiagen, Hilden Germany) according to the manufacturer's specifications. The mRNA levels of selected apoptosis-related genes were determined in 5 μg total RNA per sample using the RiboQuant™ RNase protection assay kit (Pharmingen, BD Biosciences, San Diego, CA, USA) and the rat rAPO-1 multiprobe template set (Pharmingen) containing DNA templates for the synthesis of RNA probes for the detection of mRNAs encoding Fas, Bcl-x, FasL, caspase-1, caspase-3, caspase-2, Bax and Bcl-2 in comparison to mRNAs encoding two housekeeping products, L32 and GAPDH.
Immunohistology
Semiquantitative analysis of tissue sections was performed by counting AP-2α- and caspase-cleaved PARP-positive and -negative cells/nuclei in right ventricular tissue sections of three human failing hearts (IDC) and three NF control hearts. Frozen sections (5 μM) were blocked with 1% BSA for 1 h, before the anti-AP-2α antibody (10 μg/ml) was added to the slides at room temperature. After 1 h of incubation and repeated washings in PBS, the secondary DTAF-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; 1 : 200) was added for 30 min. Sections were washed again in PBS and then incubated with a monoclonal antibody against the caspase-cleaved form of PARP (0.2 μg/ml) for 1 h. The antibody was kindly provided by M Brockhaus, Roche Diagnostics, Basel, Switzerland and generated by immunization of mice with a synthetic peptide surrounding the PARP caspase cleavage site DEVD214. The antibody was specific for a caspase-generated neoepitope of the p25 fragment of PARP, but did not recognize the full-length protein.45 After washing in PBS, the secondary Cy3-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories; 1 : 300) was added to the slides for 30 min. Controls were performed with isotype-matched control antibodies. Finally, the slides were washed again, stained with DAPI and mounted in Vectashield Fluorescence Mounting Medium (Linaris, Wertheim-Bettingen, Germany). Positive and negative cells/nuclei in three to five randomly chosen microscopic fields per section of a 400-fold magnification were counted.
Statistical analysis
Data are presented as mean±S.E.M., statistical significance was tested by Student's t-test for unpaired observations with P<0.05 considered significant.
Abbreviations
- AP-2α:
-
activator protein 2α
- CSQ:
-
calsequestrin
- DAPI:
-
4′,6′-diamidino-2-phenylindole
- GFP:
-
green fluorescent protein
- IDC:
-
idiopathic-dilated cardiomyopathy
- MOI:
-
multiplicity of infection
- NF:
-
nonfailing heart
- PARP:
-
poly(ADP-ribose)polymerase
References
Cohn JN (1996) The management of chronic heart failure. N. Engl. J. Med. 335: 490–498
Ho KK, Anderson KM, Kannel WB, Grossman W and Levy D (1993) Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88: 107–115
Codd MB, Sugrue DD, Gersh BJ and Melton III LJ (1989) Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation 80: 564–572
Dec GW and Fuster V (1994) Idiopathic dilated cardiomyopathy. N. Engl. J. Med. 331: 1564–1575
Kang PM and Izumo S (2000) Apoptosis and heart failure: a critical review of the literature. Circ. Res. 86: 1107–1113
Mittmann C, Eschenhagen T and Scholz H (1998) Cellular and molecular aspects of contractile dysfunction in heart failure. Cardiovasc. Res. 39: 267–275
Williams T, Admon A, Lüscher B and Tjian R (1988) Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 2: 1557–1569
Williamson JA, Bosher JM, Skinner A, Sheer D, Williams T and Hurst HC (1996) Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics 35: 262–264
Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F, Hofstädter F, Schule R and Buettner R (1995) Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development 121: 2779–2788
Oulad-Abdelghani M, Bouillet P, Chazaud C, Dolle P and Chambon P (1996) AP-2.2: a novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp. Cell Res. 225: 338–347
Zhao F, Satoda M, Licht JD, Hayashizaki Y and Gelb BD (2001) Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J. Biol. Chem. 276: 40755–40760
Williams T and Tjian R (1991) Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 5: 670–682
Buettner R, Kannan P, Imhof A, Bauer R, Yim SO, Glockshuber R, Van Dyke MW and Tainsky MA (1993) An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol. Cell. Biol. 13: 4174–4185
Imagawa M, Chiu R and Karin M (1987) Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell 51: 251–260
Lüscher B, Mitchell PJ, Williams T and Tjian R (1989) Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 3: 1507–1517
Imhof A, Schuierer M, Werner O, Moser M, Roth C, Bauer R and Buettner R (1999) Transcriptional regulation of the AP-2alpha promoter by BTEB-1 and AP-2rep, a novel wt-1/egr-related zinc finger repressor. Mol. Cell. Biol. 19: 194–204
Mitchell PJ, Timmons PM, Hebert JM, Rigby PW and Tjian R (1991) Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 5: 105–119
Philipp J, Mitchell PJ, Malipiero U and Fontana A (1994) Cell type-specific regulation of expression of transcription factor AP-2 in neuroectodermal cells. Dev. Biol. 165: 602–614
Schorle H, Meier P, Buchert M, Jaenisch R and Mitchell PJ (1996) Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381: 235–238
Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA and Williams T (1996) Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381: 238–241
Bosher JM, Williams T and Hurst HC (1995) The developmentally regulated transcription factor AP-2 is involved in c-erbB-2 overexpression in human mammary carcinoma. Proc. Natl. Acad. Sci. USA 92: 744–747
Bosher JM, Totty NF, Hsuan JJ, Williams T and Hurst HC (1996) A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 13: 1701–1707
Gershenwald JE, Sumner W, Calderone T, Wang Z, Huang S and Bar-Eli M (2001) Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene 20: 3363–3375
Müller FU, Neumann J and Schmitz W (2000) Transcriptional regulation by cAMP in the heart. Mol. Cell. Biochem. 212: 11–17
Anon (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 353: 2001–2007
Motomura S, Deighton NM, Zerkowski HR, Doetsch N, Michel MC and Brodde OE (1990) Chronic beta 1-adrenoceptor antagonist treatment sensitizes beta 2-adrenoceptors, but desensitizes M2-muscarinic receptors in the human right atrium. Br. J. Pharmacol. 101: 363–369
Sigmund M, Jakob H, Becker H, Hanrath P, Schumacher C, Eschenhagen T, Schmitz W, Scholz H and Steinfath M (1996) Effects of metoprolol on myocardial beta-adrenoceptors and Gi alpha-proteins in patients with congestive heart failure. Eur. J. Clin. Pharmacol. 51: 127–132
The Xamoterol in Severe Heart Failure Study Group. (1990) Xamoterol in severe heart failure. Lancet 336: 1–6
Müller FU, Boheler KR, Eschenhagen T, Schmitz W and Scholz H (1993) Isoprenaline stimulates gene transcription of the inhibitory G protein alpha-subunit Gi alpha-2 in rat heart. Circ. Res. 72: 696–700
Trauth BC, Klas C, Peters AM, Matzku S, Möller P, Falk W, Debatin KM and Krammer PH (1989) Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245: 301–305
Hockenbery D, Nunez G, Milliman C, Schreiber RD and Korsmeyer SJ (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348: 334–336
Los M, Wesselborg S and Schulze-Osthoff K (1999) The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10: 629–639
Mertens PR, Steinmann K, Alfonso-Jaume MA, En-Nia A, Sun Y and Lovett DH (2002) Combinatorial interactions of p53, activating protein-2, and YB-1 with a single enhancer element regulate gelatinase A expression in neoplastic cells. J. Biol. Chem. 277: 24875–24882
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV and Gudkov AV (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285: 1733–1737
Wang G, Yeh HI and Lin JJ (1994) Characterization of cis-regulating elements and trans-activating factors of the rat cardiac troponin T gene. J. Biol. Chem. 269: 30595–30603
Garcia MA, Campillos M, Marina A, Valdivieso F and Vazquez J (1999) Transcription factor AP-2 activity is modulated by protein kinase A-mediated phosphorylation. FEBS Lett. 444: 27–31
Creaser PC, D'Argenio DA and Williams T (1996) Comparative and functional analysis of the AP2 promoter indicates that conserved octamer and initiator elements are critical for activity. Nucleic Acids Res. 24: 2597–2605
Courtois SJ, Lafontaine DA, Lemaigre FP, Durviaux SM and Rousseau GG (1990) Nuclear factor-I and activator protein-2 bind in a mutually exclusive way to overlapping promoter sequences and trans-activate the human growth hormone gene. Nucleic Acids Res. 18: 57–64
Li Z, Bing OH, Long X, Robinson KG and Lakatta EG (1997) Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am. J. Physiol. 272: H2313–H2319
Goussev A, Sharov VG, Shimoyama H, Tanimura M, Lesch M, Goldstein S and Sabbah HN (1998) Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am. J. Physiol. 275: H626–H631
Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C, Gu JL, Kumar S, Poste G, Ruffolo Jr RR and Feuerstein GZ (1998) Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ. Res. 82: 166–174
Gaubatz S, Imhof A, Dosch R, Werner O, Mitchell P, Buettner R and Eilers M (1995) Transcriptional activation by Myc is under negative control by the transcription factor AP-2. EMBO J. 14: 1508–1519
Decary S, Decesse JT, Ogryzko V, Reed JC, Naguibneva I, Harel-Bellan A and Cremisi CE (2002) The retinoblastoma protein binds the promoter of the survival gene bcl-2 and regulates its transcription in epithelial cells through transcription factor AP-2. Mol. Cell. Biol. 22: 7877–7888
Latif N, Khan MA, Birks E, O'Farrell A, Westbrook J, Dunn MJ and Yacoub MH (2000) Upregulation of the Bcl-2 family of proteins in end stage heart failure. J. Am. Coll. Cardiol. 35: 1769–1777
Bantel H, Lügering A, Poremba C, Lügering N, Held J, Domschke W and Schulze-Osthoff K (2001) Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology 34: 758–767
Lüss H, Schmitz W and Neumann J (2002) A proteasome inhibitor confers cardioprotection. Cardiovasc. Res. 54: 140–151
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW and Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95: 2509–2514
Müller FU, Boknik P, Knapp J, Neumann J, Vahlensieck U, Oetjen E, Scheld HH and Schmitz W (1998) Identification and expression of a novel isoform of cAMP response element modulator in the human heart. FASEB J. 12: 1191–1199
Jones LR, Zhang L, Sanborn K, Jorgensen AO and Kelley J (1995) Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J. Biol. Chem. 270: 30787–30796
Movsesian MA, Karimi M, Green K and Jones LR (1994) Ca(2+)-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation 90: 653–657
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Reich NC, Sarnow P, Duprey E and Levine AJ (1983) Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology 128: 480–484
Acknowledgements
The excellent technical assistance of Andrea Walter and Sabine Richter is greatly appreciated. The study was supported by the BMBF/DLR (Interdisziplinäres Zentrum für Klinische Forschung Münster, IZKF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Dr. CJ Thiele
Rights and permissions
About this article
Cite this article
Müller, F., Loser, K., Kleideiter, U. et al. Transcription factor AP-2α triggers apoptosis in cardiac myocytes. Cell Death Differ 11, 485–493 (2004). https://doi.org/10.1038/sj.cdd.4401383
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401383
Keywords
This article is cited by
-
The pro-survival function of DLEC1 and its protection of cancer cells against 5-FU-induced apoptosis through up-regulation of BCL-XL
Cytotechnology (2019)
-
Regulation of heterotrimeric G-protein signaling by NDPK/NME proteins and caveolins: an update
Laboratory Investigation (2018)
-
AP-2α expression and cell apoptosis of the lung tissue of rats with COPD and ECV304 cells stimulated by cigarette smoke extract
Chinese Science Bulletin (2011)