Abstract
Cortical neurons die in necrosis in the low-density (LD) culture, and in apoptosis in the high-density (HD) culture under the serum-free condition without any supplements. The neuronal death in LD culture was delayed by conditioned medium (CM) factors prepared from the HD culture. The CM switched the cell death mode from necrosis to apoptosis, characterized by various cell death markers and transmission electron microscopy. The CM inhibited the rapid decrease in cellular ATP levels and [3H]-2-deoxy glucose ([3H]-2-DG) uptake in the LD culture. Inhibitors of phospholipase C and protein kinase C effectively abolished the CM-induced elevation of survival activity, [3H]-2-DG uptake and ATP levels, and necrosis–apoptosis switch. All these results suggest that CM caused the cell death mode switch from necrosis to apoptosis through phospholipase C- and protein kinase C-mediated mechanisms.
Similar content being viewed by others
Introduction
Following ischemic stress of the brain, traumatic neuronal injury because of necrosis occurs in the ischemic core, while apoptosis occurs several days later at the region surrounding the core, called penumbra.1,2,3,4,5 If this pathological switch from necrosis to apoptosis has a significant role for the self-protection of postmitotic brain, apoptosis is presumed to cease the propagation of necrotic neuronal death. However, it was difficult to reproduce this mechanism in the experimental culture system, since chemically induced necrosis was generally hard to be protected or switched to apoptosis by several treatments.6,7 On the other hand, the low-glucose and anoxia treatment is widely used as an ischemic stress model in the culture of neurons.8 However, as this model induces both necrotic and apoptotic features, it is hard to discuss the cell death mode switch.
We have recently characterized the death of cultured cortical neurons in the serum-free condition without any supplements. Under this condition, most neurons rapidly died in necrosis without apoptotic features.9 When the cell density was raised five-fold, however, the cell death was delayed and the death mode was switched to apoptosis without necrotic features. For the molecular basis of mechanism of this cell death mode switch, there are two possibilities including the contribution of cell–cell contacts and soluble factors secreted in the conditioned medium (CM). Here, we attempted to demonstrate the presence of soluble factors to induce such a cell death mode switch.
Results
CM factors-induced survival of cortical neurons
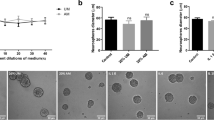
Under serum-free conditions, the survival activity of cortical neurons in the low-density culture (LD, 1 × 105 cells/cm2) rapidly decreased (Figure 1a). At 24 h after the start of culture, there was complete loss of activity. On the other hand, the decreasing rate of the activity in the high-density culture (HD, 5 × 105 cells/cm2) was much slower than that in the LD. Approximately 40% of the original activity was retained at 24 h. However, there was no significant population of 5-bromo-2′-deoxyuridine-positive cells throughout experiments (unpublished data), suggesting that the population of proliferative cells such as glia is negligible in this culture. When the CM factors prepared from the HD culture in a separate experiment was added to the LD culture at a 40% of the original CM from the beginning of culture, the decreasing rate of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt (WST-8) survival activity was slowed to the level for HD culture (Figure 1a). In the experiments measuring the death rate by use of lactate dehydrogenase (LDH) release assay kit, 28% of total LDH release in the LD culture was observed as early as 3 h from the start of culture, and reached a maximal at 24 h after the beginning of culture. However, significant LDH release was not observed at the time point of 6 h, although the release was gradually increased at later time points in HD culture. The CM treatment decreased the LDH release in the LD culture to the level similar to the HD culture (Figure 1b). Quite similar results were observed when trypan blue exclusion assay was utilized for cell death activity (Figure 1c).
Conditioned medium CM factors-induced cell survival in cultured cortical neurons under serum-free conditions. Cortical neurons were cultured in the serum-free medium under low-density (LD), high-density (HD) and LD plus CM (LD+CM). The CM-concentration-dependent survival activity was measured by WST-8 assay. Culture dishes were precoated with a 40% equivalent of CM from HD culture. The time course of survival activity was measured by WST-8 assay (a), and cell death was measured by LDH release assay (b) and trypan blue exclusion test (c). Results are expressed as the means±S.E.M. from three independent experiments. *P<0.05, compared with LD culture
CM-induced cell death mode switch
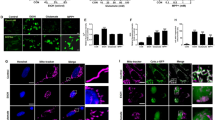
Phase-contrast microscopic observation shows that most neurons under the LD condition lost the normal thickness in morphology except nuclei 12 h after the start of culture, while those in the HD or LD plus CM conditions retained the thickness, and had typical neurites (Figure 2Aa–c). And 24 h after the start of culture, most of the neurons in the HD or LD plus CM showed a reduction in cell size, and some showed a nuclear fragmentation (Figure 2Ad–f). fluorescein isothiocyanate (FITC)-conjugated annexin V has been utilized to detect that the externalization of phosphatidylserine occurred at an early stage of apoptosis.10 On the other hand, the staining of nuclei with propidium iodide (PI) was performed to detect the plasma membrane damage, which is known to be a marker of necrosis.11 As shown in Figure 2Ba, neurons were stained with PI, but not with annexin V at the time point of as early as 3 h LD culture. As previously reported,9 on the other hand, most neurons in the HD culture were PI negative, but annexin V positive instead (Figure 2Bb). By coating with CM, PI-positive neurons were lost, while most stained ones were annexin V-positive instead (Figure 2Bc). The CM coating decreased the population of PI-positive neurons from 66.8% of total cells to 25.2%, but increased that of annexin V-positive ones from 10.1 to 55.3% (Figure 2Bd). Furthermore, in the HD condition PI-positive neurons were 16.8% and annexin V-positive neurons were 68.2% of the total cells.
CM-induced cell death mode switch from necrosis to apoptosis. (A) Phase- contrast photographs of neurons in the LD, HD and LD+CM cultures. (B) Cell death mode switch. Neurons in LD, HD and LD+CM cultures were stained with PI (red) and annexin V (FITC labeled: green) (a, b and c), PI and active caspase-3 (FITC labeled: green) (e, f and g), and PI and TUNEL (FITC labeled: green) (i, j and k) after incubation for 3, 12 and 24 h, respectively, and then observed under a confocal laser scanning microscope (confocal LSM). Quantitative comparisons of stained neurons (200–500 cells in each group) are shown with PI or annexin V (d), PI or active caspase-3 (h) and PI or TUNEL (l) between LD (L), LD+CM (C) and HD (H). Results are expressed as the means±S.E.M. from three independent experiments. *P<0.05, compared with LD culture. (C) Immunoblot analyses of protein expression of caspases-3 and -9 proteins after incubation for 12 h
The conversion of procaspase-3 to active caspase-3 is generally accepted to be a reliable indicator of apoptosis.12 As shown in Figure 2Be–h, no significant active caspase-3 signal was observed in the LD culture, while the CM coating markedly increased the signal, which is maximal 12 h after the start of culture, as well as in the HD culture.
Terminal deoxunucleotidyl transferase-mediated dUTP nick end labeled (TUNEL) activity is another indicator for apoptosis.13 This activity was maximum 24 h after the start of HD or CM plus LD culture, but not in the LD culture (Figure 2Bi–l). At this time point, however, both TUNEL and PI signals were observed, presumably because apoptotic cells begin to show membrane damages at the late stage.
The activation of caspase-3 was also confirmed by immunoblot analysis (Figure 2C). There was no significant signal at the size for activated caspase-3 cleaved from procaspase-3 in the cell lysates 0 h after the LD culture. A marked signal, however, was observed in the sample of 12 h culture in the presence of CM, although less signal was also detected in the sample of 12 h culture in its absence. Quite a similar pattern of cleavage of procaspase-9 to activated caspase-9 was also observed in these samples. However, the cleavage of procaspase-8 to activated caspase-8 was less significant and there were no significant changes among these samples (data not shown). Thus, CM factors are unlikely to drive the apoptotic signaling through tumor necrosis factor (TNF) receptor family coupled to procaspase-8 cleavage.
Electron microscopical observation of neuronal death
After the negative staining of neurons cultured for 3 or 12 h under different conditions, electron microscopical observation was carried out. There was no significant change in membrane structure, mitochondria or nucleus 3 h after the start of LD culture (Figure 3a and b). However, typical necrotic cell death features were observed in a representative neuron, in which there was decreased electron density in the cytoplasm, disrupted plasma membranes and grossly swollen mitochondria (Figure 3c and d). Swollen mitochondria had no clear cristae structures. Marked membrane disruption was observed 12 h after the LD culture. The neuron in the HD culture, on the other hand, had marked nuclear fragmentation with some condensed parts (Figure 3e), but mitochondria remained intact (Figure 3f). The neuron in the LD culture in the presence of CM also retained the electron density and had nuclear fragmentation or chromatin condensation, but no marked swollen mitochondria (Figure 3g and h).
Necrotic and apoptotic neuronal death in TEM observation. Neurons 3 h (a) or 12 h (c, e, g) after start of LD (a, c), HD (e) and LD+CM (g) cultures, respectively, were used for TEM observation. High magnification of mitochondria in the LD (b: 3 h, d: 12 h), HD (f: 12 h) and LD+CM (l: 12 h) cultures, respectively. Swollen mitochondria and intact mitochondria are indicated by arrowheads and arrows, respectively. N: nuclei
Phospholipase C mediates the action of CM factors
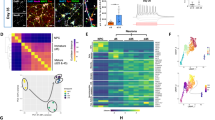
Several lines of evidence indicate that the cytosolic Ca2+ concentration can regulate apoptosis.14,15 We examined the involvement of Ca2+-related mechanisms in CM-induced neuronal survival. In the pharmacological experiments, all tested drugs affecting signal transduction, except for thapsigargin, were added to the CM-coated plate as seeding (Figure 4a). Thapsigargin is known to deplete Ca2+ from endoplasmic reticulum Ca2+ stores, including inositol.16,17 This compound concentration dependently inhibited the survival effects of CM in ranges of 0.1–10 μM. U73122, an aminosteroid inhibitor of phospholipase C (PLC),18 showed a concentration-dependent inhibition of the survival effect of CM. The concentration showing maximal inhibition and the IC50 were 10 and 1.1 μM, respectively. In contrast, the same concentration ranges of U73343, an inactive analog of U73122,19 showed no inhibitory effect on the neuronal survival by CM (data not shown). Neither verapamil, nitrendipine nor ω-conotoxin-GVIA, blockers for L and N-type Ca2+ channel, inhibited effects even at 0.1–10 μM (data not shown). These results suggest that PLC activation and the following elevation of intracellular Ca2+ concentration are associated with neuronal survival activity in CM-coated cultures.
Signal transduction for the CM-induced neuronal survival. (a) Effects of various reagents affecting Ca2+ dynamics. Cortical cells were seeded at 1 × 105 cells/cm2 onto the plate previously coated with CM (40%). Drugs were added to the culture at the beginning of the culture. Survival activity was measured by WST-8 12 h after the start of culture. Neuronal survival was expressed as percentage of inhibition of specific absorbance in CM-coated cultures. Data show mean±S.E.M. from three independent experiments. (b) Effects of various protein kinase inhibitors. Treatments of cortical cells with reagents and WST-8 were carried out, as described in (a). Neuronal survival was expressed as percentage inhibition of specific absorbance in CM-coated cultures. Data show the mean±S.E.M from three independent experiments
Effects of various protein kinase inhibitors on CM-actions
It is well known that various protein kinases affect the cell survival or cell growth. A number of growth factors possessing neurotrophic activity stimulate tyrosine kinase (TK) domain within their receptor molecules and the TK stimulation could lead to protein kinase C (PKC) activation through PLC gamma stimulation, and to phosphatidylinositol-3 kinase (PI-3 kinase) activation.20,21
Here, we examined the effects of inhibitors for various kinases on CM-induced survival. When herbimycin A, a TK inhibitor interacting with sulfhydryl groups of the kinases, at 0.1–10 μM was added to CM-coated cultures, it inhibited the survival activity of CM in a concentration-dependent manner (Figure 4b). Calphostin C (a highly selective, potent and membrane permeable PKC inhibitor)22 also concentration-dependently blocked the survival effects of CM within the ranges of 0.1–10 μM (Figure 4b). However, there was no significant inhibition by KT 5720, a specific inhibitor of cyclic AMP-dependent protein kinase (PKA),23 wortmannin, which is an inhibitor of PI-3 kinase and myosin light-chain kinase,24,25 PD98059 and U0126, inhibitors for mitogen-activated protein kinase kinase (MAPKK),26,27,28 nor by KN93, an inhibitor for Ca2+/calmodulin kinase II (data not shown).29
CM-induced survival activity has critical time in the early stage of cell culture
In order to examine the critical time, half-maximal concentration (20%) of CM was added for limited periods to the LD culture and the survival activity was measured 12 h after the start of culture (Figure 5a). Without CM treatment, the WST-8 activity was 17.7%. The CM treatment for 0–2, 0–6 or 0–12 h markedly increased the survival, but the treatment for 2–12 and 6–12 h did not. Thus, it is evident that the initial 2 h from the beginning of culture is the critical time for the CM-induced survival activity. As shown in Figure 5b, the addition of U73122 at 10 μM for the initial 2 h markedly inhibited the CM-induced survival activity to 4.4%. However, the addition of U73122 during 2–12 h showed very limited effects (68% of the control), while complete blockade was observed by the addition during 0–12 h. Quite similar results were also observed with calphostin C. On the other hand, the inhibitory effects of herbimycin A were observed only when added for long periods. Thus, the initial 2 h would be the critical period for CM-induced survival activity through PLC and PKC mechanisms.
Critical period-specific inhibition of CM-induced survival activity by U73122, calphostin C or herbimycin A. (a) Survival effects of CM treatment during various periods. CM was added to the LD culture and incubated for the periods indicated in panel A. At the end of incubation with CM, the medium was changed by the fresh one without CM. To avoid the influences of residual CM after the washout procedure, 20% equivalent CM from HD culture was used only for this paradigm of experiments. The WST-8 assay was performed 12 h after the start of culture. Data are mean±S.E.M. from three independent experiments. (b) Cortical cells were seeded 1 × 105 cells/cm2 onto the plate previously coated with 40% CM. Drugs were added to the culture during the indicated periods. Neuronal survival was expressed as percentage inhibition of specific absorbance in CM-coated cultures. Data show mean±S.E.M. from three independent experiments. *P<0.05
CM-stimulated PKC activity insensitive to herbimycin A
As shown in Figure 6, basal PKC activity was constant in vehicle-treated LD culture throughout 0–12 h after the start of culture. The CM (40%) coating of LD culture significantly increased PKC activity 2 h after the culture, while a smaller but significant increase was still observed at 12 h. However, the addition of herbimycin A at 3 μM did not affect the CM-induced increase in PKC activation (Figure 6). The addition of calphostin C at 3 μM completely abolished PKC activity in all these preparations (data not shown). Thus, it is suggested that receptor tyrosine kinases unlikely activate the CM-induced PKC activation, and thereby the inhibitory effect of U73122 is unlikely to be mediated through PLC gamma.
Calphostin C-reversible CM-induced cell death mode switch
The CM-induced cell death mode switch from necrosis to apoptosis was observed in the double staining of Hoechst 33342 and PI 12 h after the start of serum-free culture (Figure 7a). In the vehicle-treated control LD culture, most of the neurons were PI positive, but had no significant condensed or fragmented nuclei stained by Hoechst 33342, apoptosis markers. The CM coating completely diminished the population of PI-positive neurons, but increased the population showing condensed or fragmented nuclei. As shown in Figure 7a and b, further addition of calphostin C at 3 μM completely reversed the CM-induced cell death mode switch, while the addition of herbimycin A at 3 μM did not.
Calphostin C-reversible CM-induced cell death mode switch. (a and b) Double staining with Hoechst 33342 and PI 12 h after the start of serum-free culture and their quantitative comparisons. In these analyses, 500–800 cells in each group were counted. *P<0.05 compared with Hoechst 33342-stained cells
Although little is known of the mechanisms for necrosis, the decrease in cellular ATP levels is reported to cause necrosis.30 As shown in Figure 8a, the LD culture showed a rapid drop in cellular ATP levels from the time point of 30 min to 12 h after the start of serum-free culture. The speed of decrease in ATP levels of both HD and CM plus LD cultures was significantly slower than the LD culture. The question is whether ATP levels determine the cell death mode switch. To answer this question, we attempted to see the effects of oligomycin, which decreases ATP levels by inhibiting F0–F1 ATPase in mitochondria. The addition of oligomycin at 1 and 10 μM concentration dependently decreased the residual ATP levels (Figure 8b). The CM-induced increase in ATP levels was completely abolished by oligomycin at concentrations as low as 1 μM, which itself did not cause a significant decrease in the ATP levels of LD culture. Here, we used a minimum concentration of oligomycin (1 μM) for the experiments to avoid nonspecific toxicity by a higher concentration of this reagent. The treatment with 1 μM oligomycin reversed the CM-induced cell death mode switch, as seen in the increased PI staining and decreased active caspase-3 signal (Figure 8c). To examine the mechanisms underlying CM-induced inhibition of rapid decrease in ATP levels in the serum-free and LD culture, [3H]-2-deoxy glucose ([3H]-2-DG) uptake activity was measured under various conditions. The [3H]-2-DG uptake was 4444.8 dpm/well in the serum-containing LD culture, but 463.6 dpm/well in the serum-free LD culture. The CM coating increased the activity to 2744.2 dpm/well, and this increase was completely abolished by a further addition of U73122 (10 μM) or calphostin C (3 μM), as shown in Figure 8D. These inhibitors alone showed no significant effect on the uptake activity (data not shown). The influences on ATP levels by CM, U73122 and calphostin C were quite similar to the case with [3H]-2-DG uptake activity (Figure 8e). Moreover, the cytochrome c (cyto c) signal was colocalized with Mitotracker Red CMXRos, a fluorescent probe for mitochondria, 12 h after the start of serum-free culture (Figure 9), and it is evident that the apoptotic signal manifested by cyto c release is not given under the serum-free condition. The CM coating clearly induced cyto c release and this change was reversed by calphostin C at 3 μM. However, the addition of herbimycin A at 3 μM did not affect the CM-induced cyto c release (Figure 9).
Calphostin C-reversible CM-induced maintenance of intracellular ATP. (a) Time course of changes in cellular ATP levels in LD, HD and LD+CM. Relative ATP levels were calculated as the percent of the time 0 level. Results are expressed as the means±S.E.M. from three independent experiments. *P<0.05 compared with LD culture. (b) Oligomycin-reversible increases in ATP levels by CM treatment. (c) Oligomycin-reversible CM-induced switch of necrosis to apoptosis as seen in PI and active caspase-3 double staining and quantitative comparisons of stained cells. In this analysis, 500–800 cells in each group were counted. (d) Effects of U73122 and calphostin C on [3H]-2-DG uptake for 2 h immediately after the start of culture. (e) Inhibition of CM-induced increase in ATP levels by U73122 (10 μM) and calphostin C (3 μM). *P<0.05
Calphostin C-reversible cyto c release by CM. Mitochondrial membrane potential ΔΨm (CMXRos: red) and cyto c (FITC labeling: green; cyto c) staining were observed in cortical neurons treated with vehicle, CM, CM+calphostin C (3 μM) or CM+herbimycin A (3 μM) in the serum-free and LD culture. Mitochondria from vehicle- and CM+calphostin C-treated cells show a high level of staining for cyto c. In contrast, mitochondria from CM- and CM+herbimycin A-treated cells show extensive loss of cyto c staining. Combined images were produced by superimposing CMXRos (red) and cyto c (green) staining. Yellow areas are indicative of colocalized CMXRos and cyto c staining
Discussion
Initially, we found the cell density-dependent survival activity of cortical neurons in the serum-free culture, in which there were no additional supplements to increase the survival.9,31 The coating and addition of CM prepared from the HD culture markedly increased the survival activity (data not shown). These results suggest that the soluble survival factors in CM have protein-like property to adhere to the substrate, and the cells (neurons) have membrane receptors to receive signals from such CM factors. In a preliminary study, we found that proteins migrating at sizes of 20 and 22 kDa on SDS-PAGE called NDI 20 and 22 retain major survival activities, although a detailed comparison in signaling with the original CM factors remains to be determined.32
The cell density-dependent and CM-induced survival activity could be evaluated as the delay of cell death, as shown in Figure 2A. The phase-contrast microscopical analysis showed that the cells in the HD culture or those in the LD plus CM culture were healthy and extended neurites 12 h after the start of culture. However, most of the cells in such HD or LD plus CM cultures, but not in the LD culture, 24 h after the start of culture showed a reduction in cell size, and some showed a nuclear fragmentation. This difference in the cell death mode was further characterized in the sense of necrosis and apoptosis. As expected, the dead cells in the LD culture were PI positive and characterized to be necrotic ones, while those in the HD or LD plus CM culture were found to be apoptotic ones. In these experiments, necrotic cells were characterized by PI staining and broken cell membranes in transmission electron microscopy (TEM) (Figure 3c), while apoptotic cells were annexin V, activated caspase-3 and TUNEL-positive (Figures 3 and 2B). They also showed nuclear condensation and fragmentation in fluorescent microscopy after staining with Hoechst 33342, and in TEM (Figures 7a and 3). These results strongly suggest that CM factors secreted from the serum-free culture may cause the cell death mode switch from necrosis to apoptosis.
The next issue to be discussed is the molecular mechanism underlying CM-mediated survival. In many cases, Ca2+ dynamics and kinase activities are important regulators that affect cell survival.33,34 From the study using various inhibitors to affect cellular Ca2+ dynamics, PLC and its downstream Ca2+ mobilization from endoplasmic reticulum were found to be involved in CM-induced neuronal survival. Among various kinases, PKC and tyrosine kinases are likely candidates involved in these mechanisms. When the time period of neurons to be exposed to CM is varied, the critical period to induce survival activity was found to be within 2 h after the start of culture. This is also supported by data using protein kinase inhibitors, in which calphostin C, but not herbimycin A, critically inhibited CM-induced survival activity only when added for the initial 2 h. It should be noted that the CM-induced PKC activation lasts for at least 12 h. All these results suggest that PKC activation is an essential mechanism for CM-mediated survival activity, but some other unidentified mechanisms determine the critical period.
Although longer incubation with herbimycin A inhibited CM-induced survival activity, it has no effect on CM-stimulated PKC activity (Figure 6) or CM-induced cell death mode switch, characterized by the inhibition of necrosis (PI staining in Figure 7a) and induction of apoptosis (nuclear fragmentation and condensation in Figure 7a, and cyto c release in Figure 9). Moreover, the phosphorylation of PLC gamma, which is mediated by TK receptor activation, was not observed in the immunoblot analysis of cortical neurons following CM treatment (data not shown). All these results strongly suggest that the survival activities of CM showing critical period and cell death mode switch are not mediated through TK receptors. The mechanisms underlying herbimycin A-induced inhibition of survival activity independent of possessing a critical period remain to be determined.
Although little is known of the mechanisms for necrosis, the decrease in cellular ATP levels is reported to cause necrosis. In a previous report, the reduction of cellular ATP levels by oligomycin switched the Fas ligand-induced apoptosis to necrosis.31 This view is supported by the present study, in which the rapid decrease in ATP levels was observed in the LD culture, but not in the HD culture or CM plus LD culture (Figure 8a). Further analyses revealed that CM reversed the poor [3H]-2-DG uptake activity and rapid decrease in ATP levels, and these actions were abolished by calphostin C (Figure 8d and e). Thus, it is suggested that PKC activation by CM factors may activate glucose uptake and cause a subsequent increase in ATP levels. Although details of the mechanisms remain to be determined, there are some reports that PKC enhances the membrane translocation of the glucose transporter.35,36,37,38
As there was no significant cyto c release from mitochondria, a known marker event for apoptosis, in the serum-free and LD culture (Figure 9), the serum-free stress itself unlikely causes proapoptotic mechanisms. We observed an activation of caspase-3 under the condition of HD or CM plus LD culture (Figure 2B and C). As an activation of caspase-8 was not observed in such cultures (data not shown), the apoptogenic mechanisms are unlikely through a family of TNF receptors. Instead, cyto c release and caspase-9 activation were observed (Figures 9 and 2C). Thus, it is suggested that some CM factors may affect the cyto c release from mitochondria, possibly through Bax or some other related proapoptotic Bcl-2 family proteins. In addition, PKC activation may also be related to the CM-induced cyto c release or apoptosis.
In conclusion, the CM factors secreted from the HD culture caused the cell death mode switch from necrosis to apoptosis in the serum-free culture. Pharmacological analyses revealed that PKC is involved in their mechanisms for inhibition of necrosis and induction of apoptosis.
Materials and Methods
Primary culture
Primary culture of the cerebral cortex from 17 days of embryonic rats was performed according to the previously reported protocol.31,39 They were seeded onto 96-well culture dishes, eight-well Lab-Tek™ chambers, and 3.5- and 9.0-cm culture dishes that had all been coated with poly-DL-ornithine (Sigma, St. Louis, MO, USA), and cultured in DMEM/F-12 medium at 37°C in 5% CO2 atmosphere. CM was prepared from HD cultures (5 × 105 cells/cm2) 3 days after the start of culture, filtrated through a Milex filter (Millipore, Bedford, MA, USA) and frozen at −20°C until use. For CM coating, 40% of the original CM was added to culture dishes and incubated for 2 h at 25°C. The dishes were washed twice with phosphate-buffered saline (PBS) for immediate use.
LDH release assay
Cell damage was quantified by measuring activities of the cytosolic LDH released into the culture medium from damaged cells, by use of the Cytotoxicity Detection Kit from Roche Molecular Biochemicals (Mannheim, Germany) according to the manufacturer's instructions. Briefly, cells (3.2 × 103cells/100 μl/well) were seeded on a 96-well culture dish. For maximum LDH release, 100 μl of 2% Triton X-100 was added to the culture (100 μl) and incubated for 30 min at 25°C. Aliquots (100 μl) of lysates were transferred to a 96-well dish and then the reaction mixture (100 μl) from the kit was added to the wells and incubated for 90 min at 25°C in the dark. Enzyme activity was evaluated by colorimetry. For LDH release into the culture medium from cells at corresponding periods, aliquots (50 μl) of culture medium were transferred to a 96-well dish, 50 μl of 2% Triton X-100 was added, and followed by the procedures mentioned above. Results were represented as the percentage of the maximum LDH release.
WST-8 assay
To assess the viability of cells, we performed WST-8 reduction assay kit (Dojin Lab., Tokyo, Japan) according to the manufacturer's instructions. WST-8 was added to the culture for 3 h at 37°C prior to the colorimetry. The percentage of WST-8 activity was represented as the ratio of activity at different time points to that in the beginning of culture.
Trypan blue exclusion assay
To assess the neuronal cell death, an equal volume of 0.4% trypan blue was added to the culture, followed by twice washing with ice-cold PBS and fixation with 4% paraformaldehyde (PFA) in PBS for 30 min at 25°C. Stained cells were evaluated as dead ones, and results were represented by the percentage of dead cells among the total cells.
Measurement of intracellular ATP levels
Intracellular ATP levels were measured by the luciferin–luciferase method, using the ATP-Determination Kit (Molecular Probes, Eugene, OR, USA).40 Entire cells of culture including any floating cells (2 × 106 cells) were subjected to the assay. The cells were washed twice with ice-cold PBS and suspended in cell dilution buffer (4 mM EDTA, 100 mM Tris-HCl pH 7.75). Aliquots (20 μl) of cell lysates were added with the reaction solution (200 μl) containing 0.5 mM luciferin, 1.25 μg/ml luciferase and 1 mM DTT from the kit, and used for bioluminescence assay, using LUMAT LB 9507 from EG&G Berthold (Bad Wildbad, Germany).
Annexin V-binding and PI-staining assay
To assess the simultaneous observation of the early phase of apoptotic and necrotic features, cortical cells on an eight-well Lab-Tek™ chamber were treated with annexin V fluorescein (Annexin V-FLUOS Staining Kit from Roche Molecular Biochemicals) and 10 μg/ml PI for 15 min at 25°C in the dark. Cells were washed once with ice-cold PBS, fixed with 4% PFA in PBS for 30 min at 25°C and observed under a fluorescence microscope (LSM 410; Carl Zeiss, Tokyo, Japan).
Immunocytochemistry of caspase-3
Cortical cells on an eight-well Lab-Tek™ chamber were fixed with 4% PFA in PBS for 30 min, followed by permeabilization using 0.1% Triton X-100 in PBS. They were incubated in blocking buffer (2% low-fat milk powder, 2% bovine serum albumin, 0.1% Tween-20, in PBS, pH 7.4) for 1 h at 25°C. Anticleaved caspase-3 antibody (1:50; Cell Signaling, Tokyo, Japan) was added to the cells. After 2 h incubation at 25°C and washing, the cells were incubated with FITC-conjugated anti-rabbit immunoglobulin (IgG) (1 : 200; Cappel, Aurora) for 4 h at 25°C. Immunolabeled cells were observed under a fluorescence microscope.
Transmission electron microscopy
Cultured cortical neurons were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 h at 25°C. The fixed cortical neurons were postfixed with 1% osmium tetroxide for 1 h at 25°C, dehydrated in graded alcohol series, and embedded in Epon812. Ultrathin sections (80 nm thick) were cut with an Ultracut S (Leica, Austria), and then stained with uranyl acetate and lead citrate for 30 and 5 min, respectively. The stained sections were observed under an electron microscope (JEM-1210; JEOL, Tokyo, Japan).
Immunoblot analysis
SDS-PAGE electrophoresis by using 12% polyacrylamide gel and immunoblot analysis were performed as described.41 Anticaspase-8 and anti-caspase-9 antibody (1 : 500; StressGen, Victoria, Canada) and anticleaved caspase-3 antibody (1 : 500; Cell Signaling, Tokyo, Japan) were used as primary antibodies. Visualization of immunoreactive bands was performed using an enhanced chemiluminescent substrate (Super Signaling Substrate; Pierce Chemical Co., Rockford, IL, USA) for the detection of horseradish peroxides.
[3H]-2-DG uptake
[3H]-2-DG (NEN, Boston, MA, USA) uptake was measured using modified Koivisto’s methods.42 Briefly, [3H]-2-DG (1 μCi/well, 10 nM) was added to the six-well culture dishes at the beginning of culture and incubated for 2 h at 37°C in a 5%-CO2 atmosphere. Uptake of [3H]-2-DG was terminated by a rapid removal of medium, followed by twice washing with ice-cold PBS (pH 7.4). The cells were lysed in 100 μl of 0.5 M NaOH, followed by neutralization with 0.5 M HCl. The radioactivity of collected cell lysates was determined by a liquid-scintillation counter.
PKC kinase assay
Cultured cells were harvested using a solution containing 0.3% β-mercaptoethanol, 5 mM EDTA, 10 mM EGTA, 50 μg/ml PMSF, 10 mM benzamidine and 50 mM Tris-HCl (pH 7.5), homogenized on ice and added to an equal volume of glycerol. PKC assay was performed using a PKC enzyme assay system (Amersham Pharmacia Biotech, Piscataway, NJ, USA), according to the manufacturer’s instructions with modifications. Briefly, samples (S) were incubated with the substrate peptide to be phosphorylated by PKC in 50 mM Tris-HCl (pH 7.5), 30 mM dithiothreitol and [γ-32P]ATP in magnesium ATP buffer (1.2 mM ATP, 30 mM HEPES and 72 mM MgCl2, pH 7.4) for 15 min at 37°C. Maximum PKC activation (Max) was obtained by the further addition of 0.3 mg/ml L α-phosphatidyl-L-serine, 24 μg/ml phorbol 12-myristate 13-acetate and 12 mM calcium acetate in 50 mM Tris-HCl (pH 7.5). Background activation (B) was obtained by omitting substrate peptide from (S) reaction mixture. After the addition of stop solution, the reaction mixture was transferred onto peptide binding paper. These papers were washed twice with 5% acetic acid and the incorporation of 32P into peptide was determined by a liquid-scintillation counter. PKC activation was calculated by the following equation. PKC activation (%)=((S)−(B))/((Max)−(B)) × 100.
Statistical analysis
Statistical analysis was carried out using Student’s t-test following multiple comparisons of the analysis of variance (ANOVA). The criterion of significance was set at *P<0.05. All results are expressed as the mean±S.E.M.
Abbreviations
- LD:
-
low density
- HD:
-
high density
- CM:
-
conditioned medium factors
- TEM:
-
transmission electron microscopy
- PLC:
-
phospholipase C
- TK:
-
tyrosine kinase
- PKC:
-
protein kinase C
- cyto c:
-
cytochrome c
- [3H]-2-DG:
-
[3H]-2-deoxy glucose
- WST-8:
-
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium monosodium salt
- LDH:
-
lactate dehydrogenase
- FITC:
-
fluorescein isothiocyanate
- TUNEL:
-
terminal deoxunucleotidyl transferase-mediated dUTP nick end labeled
- PI:
-
propidium iodide
- PBS:
-
phosphate-buffered saline
- PFA:
-
paraformaldehyde
References
Becker KJ . (1998) Inflammation and acute stroke. Curr. Opin. Neurol. 11: 45–49
Dirnagl U, Iadecola C and Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22: 391–397
Furlan M, Marchal G, Viader F, Derlon JM and Baron JC (1996) Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann. Neurol. 40: 216–226
Linnik MD, Zobrist RH and Hatfield MD (1993) Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke 24: 2002–2008; discussion 2008–2009
Lipton P (1999) Ischemic cell death in brain neurons. Physiol. Rev. 79: 1431–1568
Nishikawa A, Sodum R and Chung FL (1992) Acute toxicity of trans-4-hydroxy-2-nonenal in Fisher 344 rats [corrected]. Lipids 27: 54–58
Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H and Lehotay DC (1997) Induction of a wide range of C(2–12) aldehydes and C(7–12) acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radical. Biol. Med. 22: 1019–1027
Moroni F, Meli E, Peruginelli F, Chiarugi A, Cozzi A, Picca R, Romagnoli P, Pellicciari R and Pellegrini-Giampietro DE (2001) Poly(ADP-ribose) polymerase inhibitors attenuate necrotic but not apoptotic neuronal death in experimental models of cerebral ischemia. Cell Death Differ. 8: 921–932
Fujita R, Yoshida A, Mizuno K and Ueda H (2001) Cell density-dependent death mode switch of cultured cortical neurons under serum-free starvation stress. Cell. Mol. Neurobiol. 21: 317–324
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM and Green DR (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182: 1545–1556
Bal-Price A and Brown GC (2000) Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J. Neurochem. 75: 1455–1464
Green DR and Reed JC (1998) Mitochondria and apoptosis. Science 281: 1309–1312
Gavrieli Y, Sherman Y and Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119: 493–501
Koike T, Martin DP and Johnson Jr EM (1989) Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc. Natl. Acad. Sci. USA 86: 6421–6425
Lipton SA and Nicotera P (1998) Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium 23: 165–171
Thastrup O, Cullen PJ, Drobak BK, Hanley MR and Dawson AP (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+–ATPase. Proc. Natl. Acad. Sci. USA 87: 2466–2470
Lupu-Meiri M, Beit-Or A, Christensen SB and Oron Y (1993) Calcium entry in Xenopus oocytes: effects of inositol trisphosphate, thapsigargin and DMSO. Cell Calcium 14: 101–110
Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ and Bunting S (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 255: 756–768
Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA and Bleasdale JE (1990) Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 253: 688–697
Heumann R (1994) Neurotrophin signalling. Curr. Opin. Neurobiol. 4: 668–679
Hetman M and Xia Z (2000) Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol. Exp. (Warsz) 60: 531–545
Kobayashi E, Nakano H, Morimoto M and Tamaoki T (1989) Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 159: 548–553
Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A and Kaneko M (1987) K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 142: 436–440
Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G and Vlahos CJ (1994) Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 54: 2419–2423
Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y and Matsuda Y (1993) Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 268: 25846–25856
Alessi DR, Cuenda A, Cohen P, Dudley DT and Saltiel AR (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270: 27489–27494
Dudley DT, Pang L, Decker SJ, Bridges AJ and Saltiel AR (1995) A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92: 7686–7689
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA and Trzaskos JM (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273: 18623–18632
Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T and Hidaka H (1991) The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 181: 968–975
Eguchi Y, Shimizu S and Tsujimoto Y (1997) Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57: 1835–1840
Sasaki Y, Fukushima N, Yoshida A and Ueda H (1998) Low-density induced apoptosis of cortical neurons is inhibited by serum factors. Cell. Mol. Neurobiol. 18: 487–496
Ueda H, Fukushima N, Yoshida A, Mizuno K and Hamabe W (2000) Neuronal death inhibitor and its signal transduction. Front. Mech. Mem. Dementia. 83–86
Grewal SS, York RD and Stork PJ (1999) Extracellular-signal-regulated kinase signalling in neurons. Curr. Opin. Neurobiol. 9: 544–553
Nozaki K, Nishimura M and Hashimoto N (2001) Mitogen-activated protein kinases and cerebral ischemia. Mol. Neurobiol. 23: 1–19
Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T and Sampson SR (2001) Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol. Cell. Biol. 21: 7852–7861
Khayat ZA, Tsakiridis T, Ueyama A, Somwar R, Ebina Y and Klip A (1998) Rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and cPKC. Am. J. Physiol. 275: C1487–1497
Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S and Kasuga M (1998) Requirement of atypical protein kinase Clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol. Cell. Biol. 18: 6971–6982
Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A and Farese RV (1999) Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J. Biol. Chem. 274: 25308–25316
Hamabe W, Fujita R, Yasusa T, Yoneda F, Yoshida A and Ueda H (2000) (–)1-(Benzofuran-2-yl)-2-propylaminopentane shows survival effect on cortical neurons under serum-free condition through sigma receptors. Cell. Mol. Neurobiol. 20: 695–702
Ha HC and Snyder SH (1999) Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 96: 13978–13982
Yoshida A, Tokuyama S, Iwamura T and Ueda H (2000) Opioid analgesic-induced apoptosis, caspase-independent cell death in human lung carcinoma A549 cells. Int. J. Mol. Med. 6: 329–335
Koivisto UM, Martinez-Valdez H, Bilan PJ, Burdett E, Ramlal T and Klip A (1991) Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J. Biol. Chem. 266: 2615–2621
Acknowledgements
We gratefully acknowledge M Niwa, S Izumi and T Koji for critical discussions, and N Fukushima and W Hamabe for technical help. Parts of this study were supported by Grants-in-Aid and Special Coordination Funds from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Y. Kuchimo
Rights and permissions
About this article
Cite this article
Fujita, R., Ueda, H. Protein kinase C-mediated necrosis–apoptosis switch of cortical neurons by conditioned medium factors secreted under the serum-free stress. Cell Death Differ 10, 782–790 (2003). https://doi.org/10.1038/sj.cdd.4401239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401239
Keywords
This article is cited by
-
Mechanistic studies of Gemcitabine-loaded nanoplatforms in resistant pancreatic cancer cells
BMC Cancer (2012)
-
Stress-induced non-vesicular release of prothymosin-α initiated by an interaction with S100A13, and its blockade by caspase-3 cleavage
Cell Death & Differentiation (2010)
-
Prothymosin-α plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition
Cell Death & Differentiation (2009)
-
Prothymosin α plays a key role in cell death mode-switch, a new concept for neuroprotective mechanisms in stroke
Naunyn-Schmiedeberg's Archives of Pharmacology (2008)
-
Real-time imaging of viable-apoptotic switch in GSNO-induced mouse thymocyte apoptosis
Apoptosis (2006)