Abstract
A new synthetic drug, benzamide riboside (BR) exhibited strong oncolytic activity against leukemic cells in the 5–10 μM range. Higher BR-concentrations (20 μM) predominantly induced necrosis which correlated with DNA strand breaks and subsequent depletion of ATP- and dATP levels. Replenishment of the ATP pool by addition of adenosine prevented necrosis and favoured apoptosis. This effect was not a pecularity of BR-treatment, but was reproduced with high concentrations of all trans-retinoic acid (120 μM) and cyanide (20 mM). Glucose was also capable to suppress necrosis and to favour apoptosis of HL-60 cells, which had been treated with necrotic doses of BR and cyanide. Apoptosis eliminates unwanted cells without affecting the microenvironment, whereas necrosis causes severe inflammation of surrounding tissues due to spillage of cell fluids into the peri-cellular space. Thus, the monitoring and maintenance of cellular energy pools during therapeutic drug treatment may help to minimize nonspecific side effects and to improve attempted drug effects.
Similar content being viewed by others
Introduction
Benzamide riboside (BR) is a C-nucleoside1 that has recently been characterised as an inosine 5′-monophosphate dehydrogenase (IMPDH)-inhibitor.2,3 This enzyme catalyses the conversion of IMP to xanthosine 5′-monophosphate (XMP) and is the rate limiting enzyme in de novo guanylate biosynthesis.4 The activity of this enzyme is significantly increased in tumour cells and therefore considered to be a potential target for cancer chemotherapy.5 Tiazofurin (TR), which is metabolized in a similar manner to that of BR, was found to inhibit the growth of human myelogenous leukaemia K5626 and human promyelocytic leukaemia HL-60 cells.7 TR is an inhibitor of IMPDH8 and Phase I/II clinical trials conducted with this compound in acute myelogenous leukaemia patients, indicated a significant reduction in leukaemic cell burden.9,10,11 BR, exhibited stronger antiproliferative activity in the K562 cells than TR12 and was shown to induce apoptosis in HL-6013 and N.1 ovarian carcinoma cells.13,14 Higher BR-concentrations however, provoked necrosis, which is a common phenomenon of pro-apoptotic drugs15,16,17 and limits chemotherapy because of non-specific drug toxicity. Overdosing results in necrosis and spilling of intracellular fluids into the peri-cellular space, leading to inflammatory responses with wide ranging destructions of surrounding tissues. Therefore it is of prime interest to develop strategies to suppress necrosis and favour apoptosis. Interestingly, both cell death modes, apoptosis and necrosis, were discussed to partly share similar early pathways.17,18,19
It was postulated that BR exerts its anti-tumour effects due to IMPDH inhibition.2,12 Therefore dGTP and other dNTP levels were analyzed and correlated with cell death modes. The necrotic trigger of high BR-concentrations was identified as DNA-clastogenic activity, which subsequently led to ATP depletion. It is likely that ATP levels are the key factor deciding the death modes, because apoptosis is energy- (ATP-) dependent, whereas necrosis is not. Here we describe a new strategy to reduce non-specific toxicity by drug-overload, by artificially keeping ATP levels high and to specifically promote apoptosis and prevent necrosis.
Results
The BR concentrations determines the type of cell death
Treatment of HL-60 cells with increasing doses of BR induced cell death which was analyzed by Trypan blue staining (data not shown).20 To further discriminate the type of cell death, the integrity of poly(ADP-ribose) polymerase [PARP] and gelsolin was examined by Western blotting (Figure 1). PARP and Gelsolin become signature-specifically fragmented by Caspase 3 upon induction of apoptosis. Addition of 0.5 μM, 1 μM, or 2 μM BR neither induced cell death nor gelsolin cleavage, whereas concentrations of 5 μM or 10 μM BR caused apoptosis which was evidenced by degradation of gelsolin into a 41 kD fragment21 and of PARP into a 89 kD fragment (Figure 1a,b). Further increase of the BR-concentrations to 20 μM led to an increase of the fraction of necrotic cells and reduced the fraction of apoptotic cells, which was also reflected by the lack of PARP and gelsolin fragmentation.
Cleavage of the caspase substrate poly(ADP-ribose) polymerase (PARP) (A) and gelsolin (B) following treatment of HL-60 cells with increasing doses of BR. Controls were treated with saline for 24 h and Western blots were performed as described in Materials and Methods. The 89 kD cleavage product of PARP and the 41 kD cleavage product of gelsolin could be detected upon treatment with 5 μM and 10 μM BR and diminished in response to higher doses of BR
HL-60 cells treated with 5 μM BR or 20 μM BR for 24 h exhibited typical apoptotic or necrotic morphologies, respectively, which was examined by electron microscopy (Figure 2). The untreated control cell (Figure 2a) was characterised by an intact cell membrane and nuclear envelope and a normal chromatin distribution. HL-60 cells treated with 5 μM BR (Figure 2b) still maintained intact membranes but electron dense chromatin marginated at the nuclear envelope as a hallmark of apoptosis. The vacuoles in the cytoplasm seemed to enlarge during BR treatment. HL-60 cells treated with 20 μM BR (Figure 2c) exhibited typical necrotic morphology such as disrupted membranes and cloudy chromatin.
Electron microscopical images of apoptotic and necrotic HL-60 cells. HL-60 cells were treated with saline (A), 5 μM BR (B), and 20 μM BR (C) for 24 h, and prepared for electron microscopoical analysis as described in Materials and Methods. Panel A shows an intact cell morphology, panel B an apoptotic cell with typical DNA condensation and margination at the nuclear envelope and panel C a necrotic cell exhibiting cloudy chromatin and destructed organelles and membranes. The bars at the lower right corners indicate 1.1 μm
Measurement of dNTP- and ATP-levels
The dNTP levels were determined after 16 h treatment. At this time point cells were still viable and membranes intact, preventing nonspecific loss of dNTPs.22
Table 1 shows that 5 μM BR and 20 μM BR repressed dGTP levels to similar extent (approximately 53% of control). The cellular dGTP concentrations did not correlate with induction of apoptosis or necrosis by 5 μM BR and 20 μM BR respectively, after 16, 24 or 48 h of treatment (compare with Figure 3a). Whereas dCTP (89% of control) – and dATP (81% of control) – levels were in the range of control when cells were treated with 5 μM BR, exposure to 20 μM BR caused a drop in dCTP and dATP levels to 37 and 33%, respectively.
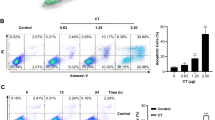
Induction of cell deaths modes by BR. (a) Cells were treated with saline (Co), 800 μM adenosine (Co+A), 5 μM BR (±adenosine), 20 μM BR (±adenosine) for 8, 16, 24 and 48 h. Then cells were harvested and stained with HO/PI, applied on glass slides, allowed to settle to the surface and then cells were counted under a microscope using a DAPI filter and cell death was determined. ‘‘e apopt’’: early apoptotic cells; ‘‘l apopt’’: late apoptotic cells; ‘‘necro’’: necrotic cells. Statistical analysis by t-test confirmed that the differences between apoptosis- and necrosis-rates after treatment with BR (±adenosine) for 48 h were significant (P<0.05). (b) Cells were treated with saline (Co), adenosine (A), 5 μM and 20 μM BR (±adenosine) for 16 h, which was a time point at which cellular membranes were still intact to avoid leaking. Then cells were harvested and the intracellular ATP content was measured as described in ‘Methods’. Statistical analysis by t-test confirmed that the differences between ATP-levels after treatment with BR (±adenosine) were significant (P<0.05). (c) Micrographs of HL-60 cells stained with Hoechst 33258 (HO) and propidium iodide (PI) after treatment with saline, 5 μM BR, 20 μM BR, and 20 μM BR+800 μM adenosine (panel A) for 48 h (1st, 2nd, 3rd and 4th slides, from left to right, respectively). Panel B shows HL-60 cells which were exposed to 55°C heat shock for increasing times. The nuclei of viable cells stain blue (the cytoplasm remains invisible). In an early phase of apoptosis condensed chromatin is visible as small round bodies which usually stain more intense blue with HO. Late apoptotic cells exhibit similar chromatin condensation but the colour shifts to pink due to PI intrusion through leaky membranes as a consequence of apoptosis progression. Upon 20 μM BR treatment, or in response to heat shock, increasing numbers of cells show a pink colour but lack apoptotic (condensed) chromatin (necrotic cells). Panel C: HL-60 cells were exposed to heat shock treatment (55°C) for 1, 3 and 5 h and PARP expression and degradation was monitored by Western blotting. There is no apoptosis specific cleavage of PARP into the 89 kD product detectable. (d) Processing of Caspase 3 into the activated p20 poly-peptide. HL-60 cells were treated with saline (control) or increasing doses of BR±800 μM adenosine. 5 μM and 10 μM BR, which induce mainly apoptosis, trigger processing of caspase 3. 20 μM and 40 μM BR mainly provokes necrosis and this is also reflected by the reduced levels of activated caspase 3. In presence of adenosine also 20 μM and 40 μM BR activate caspase 3 and induce apoptosis. Equal sample loading was controlled by Ponceau S staining
It was assumed that ATP level is a determinant of cell death modes.15,23,24,25 Hence, we determined the ATP levels in HL-60 cells (Figure 3b) after treatment with 5 μM BR (which causes apoptosis) and after exposure to 20 μM BR (which mainly causes necrosis) (Figure 3a). In case ATP determines the type of cell death, then it has to be a regulatory parameter before cell death (apoptosis and/or necrosis) occurs. Therefore, the intracellular ATP pools were examined after 16 h of treatment (Figure 3b), when the cell membranes were still intact and no non-specific loss of nucleotides took place. Treatment with a membrane permeable ATP-precursor, adenosine, was expected to replenish the intracellular pools of ATP and to inhibit necrosis. In fact, addition of 800 μM adenosine rescued the ATP levels in 20 μM BR treated cells to 31% of the control value, whereas a dramatic ATP drop to 2.7% of control was seen, when cells were exposed to 20 μM BR only (Figure 3b). In the adenosine-treated cells necrosis was indeed inhibited (Figure 3a). Figure 3c shows HOPI double-stained viable cells, early and late apoptotic cells and necrotic HL-60 cells, which were exposed to 5 μM and 20 μM BR, with or without adenosine (panel A). For reasons of comparison, panel B depicts necrotic HL-60 cells which underwent heat shock treatment (55°C) for increasing times and panel C demonstrates the lack of PARP cleavage after heat shock (Figure 3c). Re-directing necrosis to apoptosis was also reflected by Caspase 3 cleavage to its active form in presence of 800 μM adenosine in HL-60 cells which were treated with 20 μM BR (Figure 3d). Whereas Caspase 3 activation culminated (p20 kD fragment) after exposure to 5 μM and 10 μM BR after 24 h, activation was inhibited after exposure to 20 μM or 40 μM BR. In contrast, more of the activated Caspase 3-fragment was detected after 20 μM and 40 μM BR-treatment in presence of 800 μM adenosine. Lower adenosine levels had no effect probably due to limits in cellular uptake or due to specific degradation by adenosine deaminase.
ATP-, dATP-, and dCTP-levels correlate with apoptosis
To elucidate, whether a direct correlation exists between nucleotide pools and death modes, ATP, dATP and dCTP levels were plotted in combination with the corresponding apoptosis- and necrosis-rates. For improved comprehension we summarised in Table 2 the ATP-, dATP-, dCTP-levels and death data and graphically compared the inter-relationships of total cell deaths, the apoptotic- and the necrotic subtypes with nucleotide levels from differently treated cells. It can be seen in Figure 4a, b and c that ATP-, dATP-, and dCTP levels directly correlate with apoptosis rates and inversely with the necrosis rates, when cell death was induced by BR (not however in non-induced cells such as control or adenosine control; not shown in Figure 4a–c). There was no correlation of ATP or dATP levels with total cell deaths (apoptosis+ necrosis; not shown).
Correlation of nucleoside levels with cell death modes. HL-60 cells were treated with 5 μM BR (5), 20 μM BR (20), ±adenosine (+A), for 16 h, which was a time point when membranes were still intact and at which cell death modes are already determined, to measure ATP- (a), dATP- (b), and dCTP-levels (c), and for 48 h to analyze cell death modes. These graphs demonstrate, that ATP, dATP, and dCTP levels correlate directly with the percentages of apoptotic cells, whereas ATP, dATP and dCTP levels correlate indirectly with the percentages of necrotic cells
Apoptosis is an energy dependent process, because to maintain membrane integrity ATP is required. Since adenosine restored the ATP pool and prevented necrosis also glucose was anticipated to prevent necrosis18,25,26,27 and to determine death modes. In fact 100 mM glucose nearly completely inhibited necrosis of HL-60 cells induced by treatment with 20 μM BR and instead favoured apoptosis, which was determined by HOPI double-staining.13,22,28 In these experiments spontaneous cell death of controls exhibited an apoptosis : necrosis-ratio A : N=3.6 : 1. 20 μM BR resulted in a ratio A : N=1 : 3.4, which was converted by the addition of glucose to a ratio A : N=7.8 : 1.
Adenosine prevents ATRA- and KCN-triggered necrosis in favour of apoptosis
To examine, whether prevention of necrosis by energy donors was a peculiarity of BR-induced cell death, or it represents a more general mechanism, HL-60 cells were treated with all-trans retinoic acid (ATRA) and potassium cyanide (KCN). ATRA is used clinically to treat acute promyeloic leukaemia.29 KCN blocks the respiratory chain and prevents ATP generation.30,31,32 Exposure of the cells to 120 μM ATRA for 48 h resulted in 30% apoptotic and 45% necrotic cells (apoptosis : necrosis ratio A : N=1 : 1.5) (Figure 5). Addition of 100 mM glucose repressed both apoptosis and necrosis (A : N=1 : 1.2). Also 800 μM adenosine repressed both types of cell death, but in this case the apoptosis rate was increased (A : N=1.8 : 1). The effect of glucose and adenosine on KCN-induced cell death was even more dramatic: 20 mM KCN alone caused 32% apoptosis and 41% necrosis after 48 h of treatment (A : N=1 : 1.3) in HL-60 cells. Addition of 100 μM glucose did not suppress KCN-induced cell death, as in the case with ATRA, and inverted the death ratio in favour of 61% apoptosis (A : N=1.7 : 1). 800 μM adenosine substantially suppressed necrosis and increased the apoptosis rate to 43% (A : N=9.1 : 1) (Figure 5).
Modulations of cell deaths by adenosine and glucose. HL-60 cells were treated with saline (Co), 100 mM glucose (G), 800 μM adenosine (A), 120 μM all-trans retinoic acid (ATRA), 20 mM potassium cyanide (KCN) and combinations of ATRA or KCN with glucose and adenosine for 48 h. Then the type of cell death was determined by HOPI double-staining. Statistical analysis by t-test confirmed that the differences between apoptosis- and necrosis-rates versus respective controls were significant (P<0.05)
Necrotic BR-concentrations induce DNA double-strand breaks
DNA integrity was measured in individual cells with the single cell gel electrophoresis (comet) assay. The analyses were performed at time points (8 and 16 h of treatment with BR), when the cell membranes were still intact and before apoptotic or necrotic markers were observed, but when ATP- and dNTP-pools were already affected. The results of comet assays performed at neutral pH (7.5) showed that 20 μM BR, but not 5 μM BR, induced DNA double-strand breaks within 8 h of treatment. These breaks were efficiently repaired after 16 h (Figure 6a). Additional comet-analyses at alkaline pH (13.0) demonstrated also that DNA single-strand breaks occurred after incubation with 20 μM BR, but not with 5 μM BR treatment. These lesions were substantially, but not completely repaired after 16 h (Figure 6b). The ATP level after 16 h of treatment with 20 μM BR (3% control) seemed to be insufficient for the repair of the remaining DNA single-strand breaks, and this might have resulted in necrosis. These results also suggest that 5 μM BR-induced apoptosis is not causally related to DNA double- or single-strand breaks.
Induction of DNA damage in HL-60 cells by benzamide riboside (BR). Cells were treated with saline (Co), adenosine (Co+A), 5 μM, and 20 μM BR, ±adenosine, for 8 and 16 h. Then cells were harvested for comet analysis at neutral (a) and alkaline (b) pH and the extent of DNA migration was measured as described in Materials and Methods. Per experimental point three cultures were made in parallel and from each culture 50 cells were evaluated. The figures show the distribution pattern of 150 cells. The values in rectangles (b) give the per cent of cells between the dotted lines, which were treated with 20 μM BR+adenosine. The observed differences in DNA tail length between Co and 20 μM BR treatment for 8 and 16 h±adenosine, are significant under neutral and alkaline conditions
Viable and pre-apoptotic cells contain (relatively) high ATP levels in contrast to pre-necrotic cells. Therefore, we compared cells with high versus low ATP content with the extent of DNA damage after 16 h of treatment. Comet analysis at alkaline pH (13.0) revealed that the combined percentage of surviving+apoptotic cells (98% of the cells after each exposure to either 5 μM BR or 5 μM BR+adenosine) corresponded to a 25 μm DNA tail length (96 and 95%, respectively, see Table 2). Whereas the percentage of necrotic cells (51% after treatment with 20 μM BR) correlated with cells with a >25 μm DNA tail length (50%) (Table 2, Figure 6b). The inclusion of adenosine promoted DNA repair of a subset of 20 μM BR-treated cells after 16 h. However, in 26% of the 20 μM BR-damaged cells DNA-single-strand breaks also accumulated after 16 h when co-treated with adenosine, because DNA tail lengths increased (100–140 μm) (Figure 6b). The combined percentages (68%; viable+apoptotic) of 20 μM BR+adenosine treated cells did not correlate with the percentage of cells with a DNA tail length <25 μm (45%) (Table 2, Figure 6b). This demonstrates that adenosine allowed ∼23% of the cells, which still had substantially damaged DNA and that were otherwise prone for necrosis (DNA tail length >25 μm, <87 μm), to undergo apoptosis.
3-amino benzamide (3-AB) represses necrosis
Since 20 μM BR dramatically depleted the ATP pool to 3% of control we speculated that this might have been due to DNA strand break-dependent activation of poly(ADP-ribose)polymerase (PARP). PARP consumes NAD and in consequence also affects the ATP pool.33,34 Moreover, PARP activity was shown to provoke necrosis23,35,36 and in PARP(−/−) mice upon cerebral ischemia reperfusion necrotic cell death did not occur.37 3-AB is a potent inhibitor of PARP, and in fact, we found that 20 μM BR-induced necrosis was inhibited in presence of 2 mM 3-AB (Figure 7). This supports the assumption that PARP-activation might provoke BR-induced necrosis due to energy depletion, which is prevented by PARP-inhibition.
Prevention of necrosis by 3-AB. HL-60 cells were treated with saline (Co) and 20 μM BR, ±3-amino benzamide (3-AB) for 48 h. Then the cell status was analyzed by HOPI double-staining. The reduced necrosis rates after treatment with 20 μM BR+3-AB are significant (P<0.05), whereas the increased apoptosis rates are not (P=0.084)
Discussion
It is a well known phenomenon that cytotoxic drugs, which can induce apoptosis, promote necrosis when administered at higher concentrations.15,16,17,38 Several reports suggest that apoptosis and necrosis share, in part, similar (early) pathways of induction.17,18,19,39 Also p53 might determine whether a death pathway can be completed by apoptosis or whether mutated p53 allows only for a necrotic fate at equitoxic concentrations.
It was suggested that the intracellular ATP level determines whether a cell dies in an apoptotic or necrotic mode.15,23,24,25 Therefore, we investigated ATP levels in benzamide riboside (BR)-treated HL-60 cells, which apoptosed after 5 μM treatments, whereas they underwent necrosis at 20 μM BR treatment. BR is a new synthetic C-nucleoside,1 which inhibits IMPDH, the rate limiting enzyme of de novo guanylate biosynthesis. IMPDH is frequently over-expressed in cancer cells and therefore, considered a target for anti-tumour therapy. It was previously demonstrated that BR exhibits strong anti neoplastic activity in a panel of human tumour cell lines3 and was most effective in leukaemia cells by inducing apoptosis.13,14,40 The oncolytic activity of BR3 was assumed to be due to its IMPDH-inhibitory and, therefore, dGTP-limiting action.2,12 This hypothesis was strongly encouraged by the observation, that guanosine, a precursor of dGTP, prevented the oncolytic activity of BR.12,41
Our findings show, that 5 μM BR induced apoptosis, whereas 20 μM BR provoked necrosis, although both concentrations of BR inhibited dGTP synthesis to a similar degree. dGTP levels were comparably affected by adenosine, which only marginally interfered with cell survival (see Table 2). Therefore, it seems unlikely that dGTP depletion alone accounted for the cell death mechanism elicited by BR. BR-mediated limitation of dGTP levels might result in less dGTP-mediated feedback stimulation of ADP reduction by ribonucleotide reductase (RR). This in consequence decreases dADP levels and subsequently reduces dATP levels, which in fact was observed during treatment with BR. In turn, reduced dATP-mediated feedback inhibition of UDP-reduction by RR causes high dUDP and dUMP levels and this might be the reason for the observed increase in dTTP levels following BR treatment, because dUMP is the substrate for thymidylate synthase. Whereas there was no dose response correlation between ATP- and dATP-levels to total cell death, there was a direct correlation of these nucleotide pools to apoptosis and an indirect correlation to necrosis. Thus, ATP and/or dATP levels seem to determine cell death modes as it was previously suggested by others.15,24,25,39,42
In earlier investigations it was demonstrated that BR suppressed survival pathways, induced apoptosis-relevant genes13,14 and activated Caspase 8 but not Caspase 9 (Polgar et al., submitted). However, only low doses of BR (5 and 10 μM), but not high doses (20 μM and more) induced apoptosis by a pathway that culminated in Caspase 3 activation. At high BR-concentrations the majority of cell death was by necrosis presumably due to massive DNA damage. DNA double-strand breaks became rapidly repaired but a substantial amount of single-strand breaks and/or alkali-labile sites remained non-repaired. Surprisingly adenosine enabled a substantial number of the 20 μM BR-treated cells, which contained massive DNA damage (23%), to escape necrosis and to undergo an apoptotic pathway (Table 2; Figure 6b). This percentage would correspond to cells with a DNA tail length between >25 μm and <87 μm.
DNA repair processes consume energy, particularly PARP, which is an enzyme involved in DNA repair and that becomes activated in response to DNA strand breaks.43,44 PARP consumes NAD as a substrate45 and finally the cell depletes its ATP in an attempt to replenish its NAD pool.23,33,34,44,46 Thus, it is likely, that the necrotic damage arising from high BR concentrations are a consequence of DNA strand breaks and subsequent loss of ATP, which would have been required for an orchestrated apoptotic programme. Since maintenance of ATP by adenosine or supplementation with glucose, which is the major energy source of a cell, could prevent necrosis and favoured apoptosis, therefore, ATP and possibly also dATP are determinants of cell death modes.
This investigation supports the assumption, that energy donors can promote apoptosis and repress necrosis when cell death is induced by various cytotoxic and therapeutic agents.
Materials and Methods
Chemicals
BR was synthesized as described earlier.1 Polyclonal Caspase 3 antibody was purchased from Research Diagnostics (Flanders, NJ, USA), adenosine, glucose, 3-amino benzamide (3-AB), all trans retinoic acid (ATRA) and cyanide (KCN) were from Sigma (St. Louis, MO, USA).
Cell culture
The HL-60 human acute promyelocytic leukaemia cell line was from ATCC (Rockville, MD, USA). Cells were grown in RPMI 1640 medium (Gibco, Grand Island, NY, USA) with 10% heat inactivated FCS (Boehringer Mannheim GmbH, Mannheim, Germany) and 2 mM L-Glutamine (Gibco, Gaithersburg, MD, USA) in humidified atmosphere with 5% CO2 at 37°C.
Hoechst 33258 propidium iodide (HOPI) double-staining
Hoechst 33258 (HO; Sigma) and propidium iodide (PI; Sigma) were added directly to the culture medium to final concentrations of 5 μg/ml and 2 μg/ml, respectively. After an incubation period of 1 h at 37°C, the cells were examined under a Zeiss Axiovert 35 fluorescence microscope with DAPI filters. Cells were photographed on Kodak Ektachrome P1600 film (Eastman Kodak Company, Rochester, NY, USA) and viable, apoptotic, and necrotic cells were counted manually. The Hoechst dye stains the nuclei of all cells and therefore allows to monitor nuclear changes associated with apoptosis, such as chromatin condensation and nuclear fragmentation (Figure 3c). PI, on the other hand, is excluded from viable and early apoptotic cells, consequently PI uptake indicates loss of membrane integrity characteristic of necrotic and late apoptotic cells. In combination with fluorescence microscopy, the selective uptake of the two dyes allows the monitoring of the induction of apoptosis in intact cultures and to distinguish it from non-apoptotic cell death (necrosis). Necrosis is characterised in this system by nuclear PI uptake without chromatin condensation or nuclear fragmentation.
Electron microscopy
For transmission electron microscopy (TEM) cells treated with PBS (controls), 5 or 20 μM BR for 24 h were fixed with 2.5% glutaraldehyde (in 0.1 M sodium cacodylate buffer with 4% sucrose, pH 7.2) for 45 min, washed in sodium cacodylate buffer and post-fixed in 2% osmium tetroxide (in 0.1 M sodium cacodylate buffer with 4% sucrose) for 45 min. Following several washes, the cells were concentrated by centrifugation at 150 g for 5 min, dehydrated in a graded series of ethanol, washed in propylene oxide, embedded in Epon (Serva, Germany) and sectioned at about 70 nm. The ultra-thin sections were stained with uranyl acetate/lead citrate for observation with a Zeiss EM 902 transmission electron microscope.
ATP assay
ATP content was measured with the ATP bioluminescence assay kit HS II from Boehringer Mannheim (Roche Molecular Biochemicals, Mannheim, Germany). Cells were treated with adenosine and BR for 16 h, subsequently their viability was measured by Trypan blue exclusion. There were <10% dead cells in each sample. 2.5 million cells were pelleted for each measurement and resuspended in 50 μl dilution buffer. Equal amount of cell lysis reagent was then added and after an incubation period of 5 min at room temperature the samples were transferred to microtiter plates. Luciferase reagent (100 μl) was added and the signal was detected immediately on a Lumi Imager F1 (Roche). Experiments were done in triplicates and the values of treated samples calculated as per cent of untreated controls.
Western blots
Cells from treated and untreated cultures were sedimented, washed in cold PBS, and lysed in SDS sample buffer (25 mM TRIS pH 6.8, 3% SDS, 10% glycerol, 36 mM DTT, 0.925 mM EDTA). Equal amounts of protein (calculated with the Dot Metric Protein Assay Kit from Novus Molecular, San Diego, CA, USA) were loaded onto 10 or 15% polyacrylamide gels. Proteins were electrophoresed at 80 V for 2–3 h and then transblotted onto PVDF membranes (Hybond P, Amersham International, UK) at 80 V for 2 h.47 Membranes were quenched in PBS with 0.5% skim milk and 0.05% Tween 20 for 1 h, incubated with primary antibodies (mouse monoclonal anti PARP C-2-10 used 1 : 2000, mouse monoclonal anti gelosin used 1 : 2000, Sigma; rabbit polyclonal anti caspase 3 antibody used 1 : 1000, Research Diagnostics) overnight at 4°C and with horse radish peroxidase conjugated secondary antibody for 2 h at room temperature. The ECL Kit (Amersham International, UK) was used for blot development, chemiluminescence was detected on Kodak Xomat UV films.
Deoxyribonucleotide extraction and measurement
dNTPs were extracted as described previously48 with trichloroacetic acid (TCA) 10% final concentration) followed by neutralization by trioctylamine and 1,1,2-trichlorotrifluoroethane (1 : 4) mixture. The TCA extract was dried using a Speedvac drying system at room temperature and, if necessary, stored at −20°C until analysis. The assay for dNTP, which is based on the original DNA-polymerase assay,49 was optimized by the use of 96-well plates50 and tailor-made oligonucleotides,51,52 and was performed as previously described for dCTP.48 After reconstitution of the samples in HEPES-buffered assay buffer (pH 7.3) at 107 cells/ml, samples and standards (0, 1, 2.5 and 5 pmol dNTP) were added to diethylaminoethyl (DEAE) filter plates (Millipore, Ettenleur, The Netherlands). This was followed by addition of demi water (up to 30 μl) and 70 μl of a reaction mix, consisting of 10 μl [8-3H]dATP (25 μM; 1.6 Ci/mmol; 0.04 μCi/μl) for detection of dCTP, dTTP and dGTP and 10 μl [CH3-3H]dTTP (25 μM, 1.6 Ci/mmol, 0.04 μCi/μl) for dATP detection, 5 μl appropriate oligonucleotide (6 μM, consisting of a primer attached to one of four possible templates specially designed for the detection of one of the four dNTPs), 5 μl Klenow DNA pol I and 50 μl assay buffer. The filterplates were gently vortexed and incubated at room temperature for 2 h. The wells were washed, the wet filters punched out and radioactivity counted as described.48
Comet assay and statistical analysis
Neutral and alkaline single cell gel electrophoresis (SCGE) assays were carried out following the protocol described by Singh et al.53 To measure DNA double-strand breaks electrophoresis was carried out under neutral conditions at pH 7.5,54 to analyze single-strand breaks the electrophoresis was carried out under alkaline conditions at pH 13.0.55 HL-60 cells were treated with BR and adenosine for 16 h, then the viability of the cells was determined with Trypan blue. All cultures which were used for Comet analysis had a viability of >90%. Pellets obtained upon centrifugation were mixed with 100 μl low melting agarose (0.5%, 37°C) and spread on agarose coated slides according to Klaude et al.56 Subsequently, the slides were exposed to lysis buffer53 and transferred to neutral and alkaline electrophoresis buffer respectively for 40 min to allow unwinding of DNA. Thereafter, electrophoresis was carried out for 40 min (300 mA, 25 V) at pH 7.5 and 13.0 respectively. Finally the slides were stained with ethidium bromide and evaluated under a fluorescence microscope (Nikon Model: 027012) with an automated image analysis system.57 For each experimental point, three cultures were evaluated and from each culture the tail lengths of 50 cells were determined. Statistically significant (P<0.05) differences were determined with one way ANOVA.57
Abbreviations
- ATP:
-
adenosine triphosphate
- ATRA:
-
all-trans retinoic acid
- BR:
-
3-(1-β-D-ribofuranosyl)benzamide or benzamide riboside
- dATP:
-
2′-deoxyadenosine 5′-triphosphate
- dGTP:
-
2′-deoxyguanosine 5′-triphosphate
- DTT:
-
DL-dithiothreitol
- EDTA:
-
ethylenediaminetetraacetic acid
- FCS:
-
foetal calf serum
- GTP:
-
guanosine 5′-triphosphate
- IMP:
-
inosine 5′-monophosphate
- IMPDH:
-
inosine 5′-monophosphate dehydrogenase
- HO:
-
Hoechst 33258
- KCN:
-
potassium cyanide
- NAD:
-
nicotinamide adenine dinucleotide
- PARP:
-
poly(ADP-ribose)polymerase
- PBS:
-
phosphate-buffered saline
- PI:
-
propidium iodide
- RR:
-
ribonucleotide reductase
- SCGE:
-
single cell gel electrophoresis or comet assay
- SDS:
-
sodium dodecyl sulfate
- TCA:
-
trichloro acetic acid
- TEM:
-
transmission electron microscopy
- TR:
-
2-β-D-ribofuranosylthiazole-4-carboxamide or tiazofurin
- XMP:
-
xanthosine 5′-monophosphate
References
Krohn K, Heins H, Wielckens K . 1992 Synthesis and cytotoxic activity of C-glycosidic nicotinamide riboside analogues J. Med. Chem. 35: 511–517
Jayaram HN, Gharehbaghi K, Jayaram NR, Rieser J, Krohn K, Paull KD . 1992 Cytotoxicity of a new IMP dehydrogenase inhibitor, benzamide riboside, to human myelogenous leukemia K562 cells Biochem Biophys Res Commun 186: 1600–1606
Gharehbaghi K, Sreenath A, Hao Z, Paull KD, Szekeres T, Cooney DA, Krohn K, Jayaram HN . 1994 Comparison of biochemical parameters of benzamide riboside, a new inhibitor of IMP dehydrogenase, with tiazofurin and selenazofurin Biochem. Pharmacol. 48: 1413–1419
Jackson RC, Weber G, Morris HP . 1997 IMP dehydrogenase, an enzyme linked with proliferation and malignancy Nature 256: 331–333
Weber G . 1983 Biochemical strategy of cancer cells and the design of chemotherapy: G.H.A. Clowes Memorial Lecture Cancer Res. 43: 3466–3492
Jayaram HN, Zhen W, Gharehbaghi K . 1993 Biochemical consequences of resistance to tiazofurin in human myelogenous leukemic K562 cells Cancer Res. 53: 2344–2348
Knight RD, Mangum J, Lucas DL, Cooney DA, Khan EC, Wright DG . 1987 Inosine monophosphate dehydrogenase and myeloid cell maturation Blood 69: 634–639
Jayaram HN, Dion RL, Glazer RI, Johns DG, Robins RK, Srivastava PC, Cooney DA . 1982 Initial studies on the mechanism of action of a new oncolytic thiazole nucleoside, 2-beta-D-ribofuranosylthiazole-4-carboxamide (NSC 286193) Biochem. Pharmacol. 31: 2371–2380
Tricot GJ, Jayaram HN, Lapis E, Natsumeda Y, Nichols CR, Kneebone P, Heerema N, Weber G, Hoffman R . 1989 Biochemically directed therapy of leukemia with tiazofurin, a selective blocker of inosine 5′-phosphate dehydrogenase activity Cancer Res. 49: 3696–3701
Wright DG, Boosalis MS, Waraska K, Oshry LJ, Weintraub LR, Vosburgh E . 1996 Tiazofurin effects on IMP-dehydrogenase activity and expression in the leukemia cells of patients with CML blast crisis Anticancer Res. 16: 3349–3351
Jayaram HN, Lapis E, Tricot G, Kneebone P, Paulik E, Zhen W, Engeler GP, Hoffman R, Weber G . 1992 Clinical pharmacokinetic study of tiazofurin administered as a 1-hour infusion Int. J. Cancer 51: 182–188
Gharehbaghi K, Paull KD, Kelley JA, Barchi JJ Jr, Marquez VE, Cooney DA, Monks A, Scudiero D, Krohn K, Jayaram HN . 1994 Cytotoxicity and characterization of an active metabolite of benzamide riboside, a novel inhibitor of IMP dehydrogenase Int. J. Cancer 56: 892–899
Jayaram HN, Cooney DA, Grusch M . 1999 Consequences of IMP dehydrogenase inhibition, and its relationship to cancer and apoptosis Curr. Med. Chem. 6: 561–574
Grusch M, Rosenberger G, Fuhrmann G, Braun K, Titscher B, Szekeres T, Fritzer-Skekeres M, Oberhuber G, Krohn K, Hengstschlaeger M, Krupitza G, Jayaram HN . 1999 Benzamide riboside induces apoptosis independent of Cdc25A expression in human ovarian carcinoma N.1 cells Cell Death Differ. 6: 736–744
Tsujimoto Y . 1997 Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes Cell Death Differ. 4: 429–434
Nicotera P, Leist M, Ferrando-May E . 1998 Intracellular ATP, a switch in the decision between apoptosis and necrosis Toxicol. Lett. 102: 139–142
Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ . 2000 Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis J. Cell. Physiol. 182: 41–49
Leist M, Nicotera P . 1997 The shape of cell death Biochem. Biophys. Res. Commun. 236: 1–9
Samali A, Nordgren H, Zhivotovsky B, Peterson E, Orrenius S . 1999 A comparative study of apoptosis and necrosis in HepG2 cells: oxidant-induced caspase inactivation leads to necrosis Biochem. Biophys. Res. Commun. 255: 6–11
Rosenberger G, Fuhrmann G, Grusch M, Fassl S, Elford HL, Smid K, Peters GJ, Szekeres T, Krupitza G . 2000 The ribonucleotide reductase inhibitor trimidox induces c-myc and apoptosis of human ovarian carcinoma cells Life Sci. 67: 3131–3142
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT . 1997 Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis Science 278: 294–298
Grusch M, Fritzer-Szekeres M, Fuhrmann G, Rosenberger G, Luxbacher C, Elford HL, Smid K, Peters GJ, Szekeres T, Krupitza G . 2001 Activation of caspases and induction of apoptosis by amidox and didox Exp. Hematol. in press
Ha HC, Snyder SH . 1999 Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion Proc. Natl. Acad. Sci. USA 96: 13978–13982
Nicotera P, Leist M . 1997 Energy supply and the shape of death in neurons and lymphoid cells Cell Death Differ. 4: 435–442
Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P . 1997 Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis J. Exp. Med. 185: 1481–1486
Leist M, Single B, Naumann H, Fava E, Simon B, Kuhnle S, Nicotera P . 1999 Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis Exp. Cell. Res. 249: 396–403
Petronini PG, Urbani S, Alfieri R, Borghetti AF, Guidotti GG . 1996 Cell susceptibility to apoptosis by glutamine deprivation and rescue: survival and apoptotic death in cultured lymphoma-leukemia cell lines J. Cell. Physiol. 169: 175–185
Fritzer-Szekeres M, Grusch M, Luxbacher C, Horvath S, Krupitza G, Elford HL, Szekeres T . 2000 Trimidox, an inhibitor of ribonucleotide reductase, induces apoptosis and activates caspases in HL-60 promyelocytic leukemia cells Exp. Hematol. 28: 924–930
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L . 1990 All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results Blood 76: 1704–1709
Fernandez PA, Rotello RJ, Rangini Z, Doupe A, Drexler HC, Yuan J . 1994 Expression of a specific marker of avian programmed cell death in both apoptosis and necrosis Proc. Natl. Acad. Sci. USA 91: 8641–8645
Nicotera P, Thor H, Orrenius S . 1989 Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia FASEB J. 3: 59–64
Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y . 1996 Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors Oncogene 13: 21–29
Lee YJ, Shacter E . 1999 Oxidative stress inhibits apoptosis in human lymphoma cells J. Biol. Chem. 274: 19792–19798
Filipovic DM, Meng X, Reeves WB . 1999 Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells Am. J. Physiol. 277: F428–436
Kim JW, Won J, Sohn S, Joe CO . 2000 DNA-binding activity of the N-terminal cleavage product of poly(ADP-ribose) polymerase is required for UV mediated apoptosis J. Cell. Sci. 113: 955–961
Watson AJ, Askew JN, Benson RS . 1995 Poly(adenosine diphosphate ribose) polymerase inhibition prevents necrosis induced by H2O2 but not apoptosis Gastroenterology 109: 472–482
Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL . 1997 Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia Nat. Med. 3: 1089–1095
Finstad HS, Kolset SO, Holme JA, Wiger R, Farrants AK, Blomhoff R, Drevon CA . 1994 Effect of n-3 and n-6 fatty acids on proliferation and differentiation of promyelocytic leukemic HL-60 cells Blood 84: 3799–3809
Eguchi Y, Shimizu S, Tsujimoto Y . 1997 Intracellular ATP levels determine cell death fate by apoptosis or necrosis Cancer Res. 57: 1835–1840
Hunakova L, Duraj J, Romanova D, Novotny L, Sedlak J, Kelley MR, Szekeres T, Jayaram HN, Chorvath B . 1998 Staurosporine enhanced benazmide riboside-induced apoptosis in human multidrug-resistant promyelocytic leukemia cells (HL-60/VCR) in vitro Neoplasma 45: 204–209
Vitale M, Zamai L, Mazzotti G, Cataldi A, Falcieri E . 1993 Differential kinetics of propidium iodide uptake in apoptotic and necrotic thymocytes Histochemistry 100: 223–229
Lieberthal W, Menza SA, Levine JS . 1998 Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells Am. J. Physiol. 274: F315–327
Juarez-Salinas H, Sims JL, Jacobson MK . 1979 Poly(ADP-ribose) levels in carcinogen-treated cells Nature 282: 740–741
Wielckens K, Schmidt A, George E, Bredehorst R, Hilz H . 1982 DNA fragmentation and NAD depletion. Their relation to the turnover of endogenous mono(ADP-ribosyl) and poly(ADP-ribosyl) proteins J. Biol. Chem. 257: 12872–12877
Ueda K, Hayaishi O . 1985 ADP-ribosylation Annu. Rev. Biochem. 54: 73–100
Althaus FR, Richter C . 1987 ADP-ribosylation of proteins. Enzymology and biological significance Mol. Biol. Biochem. Biophys. 37: 1–237
Towbin H, Staehelin T, Gordon J . 1979 Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications Proc. Natl. Acad. Sci. USA 76: 4350–4354
van Moorsel CJ, Pinedo HM, Veerman G, Guechev A, Smid K, Loves WJ, Vermorken JB, Postmus PE, Peters GJ . 1999 Combination chemotherapy studies with gemcitabine and etoposide in non-small cell lung and ovarian cancer cell lines Biochem. Pharmacol. 57: 407–415
Hunting D, Henderson JF . 1981 Determination of deoxyribonucleoside triphosphates using DNA polymerase: a critical evaluation Can. J. Biochem. 59: 723–727
Peters GJ, De Abreu RA, Oosterhof A, Veerkamp JH . 1983 Concentration of nucleotides and deoxynucleotides in peripheral and phytohemagglutinin-stimulated mammaliam lymphocytes. Effects of adenosine and deoxyadenosine Biochim. Biophys. Acta 759: 7–15
Sherman PA, Fyfe JA . 1989 Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers Anal. Biochem. 180: 222–226
Iwasaki H, Huang P, Keating MJ, Plunkett W . 1997 Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells Blood 90: 270–278
Singh NP, McCoy MT, Tice RR, Schneider EL . 1988 A simple technique for quantitation of low levels of DNA damage in individual cells Exp. Cell Res. 175: 184–191
Olive PL, Wlodek D, Banath JP . 1991 DNA double-strand breaks measured in individual cells subjected to gel electrophoresis Cancer Res. 51: 4671–4676
Tice RR, Strauss GH . 1995 The single cell gel electrophoresis/comet assay: a potential tool for detecting radiation-induced DNA damage in humans Stem Cells 13 Suppl 1: 207–214
Klaude M, Eriksson S, Nygren J, Ahnstrom G . 1996 The comet assay: mechanisms and technical considerations Mutat. Res. 363: 89–96
Uhl M, Helma C, Knasmuller S . 2000 Evaluation of the single cell gel electrophoresis assay with human hepatoma (Hep G2) cells Mutat. Res. 468: 213–225
Acknowledgements
We wish to thank Anton Jäger for his excellent technical assistance in preparing the figures. Grant support: This work was supported in part by the Herzfelder'sche Familienstiftung, the AICR-grant No. 97-12, the Anton Dreher Memorial Foundation to G Krupitza and by Austrian National Bank Fonds No. 8260 and the Hochschuljubiläumsfonds to T Szekeres.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by RA Knight
Rights and permissions
About this article
Cite this article
Grusch, M., Polgar, D., Gfatter, S. et al. Maintenance of ATP favours apoptosis over necrosis triggered by benzamide riboside. Cell Death Differ 9, 169–178 (2002). https://doi.org/10.1038/sj.cdd.4400937
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400937
Keywords
This article is cited by
-
Alterations in metabolic flux in migraine and the translational relevance
The Journal of Headache and Pain (2022)
-
Modelling human CNS injury with human neural stem cells in 2- and 3-Dimensional cultures
Scientific Reports (2020)
-
Hexane soluble extract of Mallotus philippensis(Lam.) Muell. Arg. root possesses anti-leukaemic activity
Chemistry Central Journal (2013)
-
In vitro inhibition of breast cancer spheroid-induced lymphendothelial defects resembling intravasation into the lymphatic vasculature by acetohexamide, isoxsuprine, nifedipin and proadifen
British Journal of Cancer (2013)
-
Inhibition of tumour spheroid-induced prometastatic intravasation gates in the lymph endothelial cell barrier by carbamazepine: drug testing in a 3D model
Archives of Toxicology (2013)