Abstract
The relationship is investigated between mitochondrial membrane potential (ΔΨM), respiration and cytochrome c (cyt c) release in single neural bcl-2 transfected cells (GT1-7bcl-2) or GT1-7puro cells during apoptosis induced by staurosporine (STS). Bcl-2 inhibited the mitochondrial release of cyt c and apoptosis. Three different cell responses to STS were identified in GT1-7puro cells: (i) neither ΔΨM nor cyt c were significantly affected; (ii) a decrease in ΔΨM was accompanied by a complete release of cyt c; or (iii) cyt c release occurred independently of a loss of ΔΨM. The endogenous inner membrane proton leak of the in situ mitochondria, monitored by respiration in the presence of oligomycin, was increased by STS by 92% in puro cells, but by only 23% in bcl-2 cells. STS decreased respiratory capacity, in the presence of protonophore, by 31% in puro cells and by 20% in bcl-2 cells. In the absence of STS, oligomycin hyperpolarized mitochondria within both puro and bcl-2-transfected cells, indicating that the organelles were net generators of ATP. However after 15 h exposure to STS oligomycin rapidly collapsed residual mitochondrial polarization in the puro cells, indicating that ΔΨM had been maintained by ATP synthase reversal. bcl-2 cells in contrast, maintained ΔΨM until protonophore was added. These results indicate that the maintenance of ΔΨM following release of cyt c may be a consequence of ATP synthase reversal and cytoplasmic ATP hydrolysis in STS-treated GT1-7 cells. Cell Death and Differentiation (2001) 8, 995–1003
Similar content being viewed by others
Introduction
In recent years, mitochondria have become centrally implicated in the process of apoptotic cell death.1,2 Release of mitochondrial cytochrome c (cyt c) is promoted by the pro-apoptotic proteins Bax and Bak, whereas anti-apoptotic members of this family prevent the release of the cytochrome.3 The mechanisms responsible for cyt c release are controversial; both non-specific rupture of the outer membrane, as a consequence of matrix swelling induced by the mitochondrial permeability transition, and activation of a specific outer membrane channel, perhaps involving the outer membrane porin, have been proposed.4 While it is unreasonable to expect that a single global mechanism will account for all observations, a key discriminator has been the mitochondrial membrane potential, ΔΨM, during the apoptotic process, since a permeability transition would be associated with a collapse in potential, while activation of a specific outer membrane channel need not involve any bioenergetic disruption. Unfortunately, a number of studies have used inappropriate methodologies to monitor ΔΨM5 and the central question concerning the bioenergetic competence of the mitochondria to generate ATP has generally not been addressed.
Exposure to the non-selective protein kinase inhibitor staurosporine (STS) induces apoptosis in a variety of cells;6,7,8,9,10 however the mechanism is still largely unknown. Heiskanen et al.8 reported that STS-induced apoptosis in PC6 cells was associated with a synchronous release of cyt c (as a green-fluorescent-protein construct) and collapse of ΔΨM. However other investigators have found that the release of cyt c into the cytosol during STS-induced apoptosis occurs independently of mitochondrial depolarization.9,10,11
The cell death repressor protein Bcl-2 inhibits cellular free radical formation12,13,14 and cyt c release,15 blocks the activation of caspases16 and inhibits apoptosis.17 Bcl-2 increases the capacity of mitochondria to accumulate Ca2+ 18 and shifts the redox potential of cells towards reduction.19 Vander Heiden et al.6 have reported that Bcl-x(L)-expressing cells adapt to STS treatment by maintaining a decreased ΔΨM, while Shimizu et al.20 have suggested that Bcl-2 over-expression decreases the effectiveness of protonophores in depolarizing the mitochondria by enhancing ion flux.
Mitochondrial membrane potential can be controlled by proton leakage across the inner membrane, ATP synthesis (or hydrolysis), substrate availability, electron flux through the respiratory chain and ion transport. In order to establish which, if any, of these factors influence ΔΨM during STS exposure we have investigated changes in ΔΨM and cyt c retention in single neural GT1-7 cells transfected with a recombinant retrovirus carrying bcl-2 and a puromycin resistance gene (GT1-7bcl-2) or a control construct (GT1-7puro) during apoptosis induced by STS. We suggest that some of the ambiguity in the literature may stem from the ability of mitochondria that have released cyt c to maintain a high ΔΨM by ATP synthase reversal.
Results
Bcl-2 expression, STS-mediated apoptosis and cyt c release
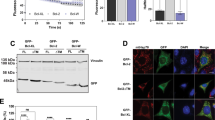
STS-induced apoptosis of GT1-7 cells is accompanied by caspase activation, nuclear fragmentation and DNA laddering.16 Figure 1 shows that the cells display other characteristic changes including externalisation of phosphatidylserine (PS) (Figure 1A) and loss of cyt c-like immuno-activity from mitochondria (Figure 1C) and cells (Figure 1B). GT1-7puro cells do not express detectable amounts of Bcl-2 (Figure 1C), but expression of the protein in the GT12-7bcl-2 cells (Figure 1C) confers protection against STS-induced apoptosis, preventing PS externalization (Figure 1A), nuclear condensation and cell death (Figure 1A) and cyt c depletion from mitochondria (Figure 1C) and cells (Figure 1B).
STS-induced apoptosis and mitochondrial cyt c release in GT1-7 cells – effect of Bcl-2. (A) The cells (GT1-7puro and GT1-7bcl-2) were incubated with 0.5 μM STS for the indicated period and imaged for phosphatidylserine exposure by labeling with Annexin V plus propidium iodide (PI) or for chromatin condensation with SYTO-13 (green) plus PI (red). Co-localization of SYTO-13 plus PI indicates necrotic or late apoptotic cells. Images are representative from 3–4 independent experiments. (B) Control cells or cells exposed to 0.5 μM STS for 15 h were co-labeled with anti-cyt c antibody and MitoTracker-green (MT-g). Overlay of fluorescence indicates retention of mitochondrial cyt c in bcl-2 cells treated with STS. (C) Immunoblots of cytoplasmic Bcl-2 expression and mitochondrial cyt c in control cells and cells exposed to 0.5 μM STS for 15 h
Mitochondrial membrane potential and cyt c retention
Changes in in situ mitochondrial membrane potential can be monitored by confocal microscopy using fluorescent cationic probes loaded at low concentrations insufficient to induce aggregation and fluorescence quenching within the matrix. Tetramethylrhodamine methyl ester (TMRM+) loaded at 10 nM into a field of GT1-7puro cells that had been exposed to 500 nM STS for 15 h revealed a heterogeneous response, with many cells retaining TMRM+ indicating a population of polarized mitochondria (Figure 2B). Mitochondria within GT12-7bcl-2 cells similarly exposed to STS were uniformly polarized (data not shown). Since Figure 1 showed extensive cyt c depletion in STS-exposed puro cells it was important to establish the relationship between ΔΨM and cyt c retention in individual cells. This was accomplished by fixing and immuno-staining the same field of cells whose mitochondrial membrane potential had been monitored. Figure 2C shows that only a sub-population of cells displaying TMRM+ fluorescence were immuno-reactive for cyt c (see for example cells arrowed ‘c’). Thus mitochondria can lose their cyt c during apoptosis by a mechanism independent of a collapsed ΔΨM. Figure 2D–F shows a group of four cells enlarged from Figure 2A–C. All four cells maintain TMRM+ fluorescence, but cell ‘b’ contains no detectable cyt c immuno-activity.
TMRM+ fluorescence and cyt c immunoactivity in puro cells treated with STS. Cells preincubated with 0.5 μM STS for 12 h were equilibrated with 10 nM for 30 min, imaged for TMRM+ fluorescence, fixed without disturbing the field and labeled with monoclonal cyt c antibody and secondary antibody conjugated with FITC. (A) bright-field image; (B) TMRM+ fluorescence; (C) cyt c staining of the same field. Note the presence of three distinct responses to STS: a, a cell that shows complete mitochondrial depolarization and is not labeled for cyt c; b, a cell that retains ΔΨM even though cyt c has been released; .c, a cell that retains both ΔΨM and cyt c. (D–F) enlargement of the insert. The bar represents 10 μm. Results are representative of five independent experiments
Since electron transfer between mitochondrial complexes III and IV is catalysed by cyt c the question is raised as to how ΔΨM is maintained in cells whose mitochondria have released the cytochrome. In the absence of electron transport the only mechanism for generating and maintaining ΔΨM is reversal of the ATP synthase utilizing cytoplasmic ATP derived from glycolysis. The ATP synthase inhibitor oligomycin has proved a useful tool to investigate ATP synthase directionally in cultured neurons undergoing excitotoxic degeneration.21,22 Briefly, by blocking proton re-entry through an ATP synthase engaged in net ATP synthesis, oligomycin will hyperpolarize the mitochondria. Alternatively, mitochondria with inhibited respiration or a moderately proton-leaky inner membrane can still maintain a ΔΨM by ATP synthase reversal, in which case oligomycin will rapidly depolarize the mitochondria.
Assessing the functional ability of in situ mitochondria to generate ATP is of more value than a simple qualitative estimate of mitochondrial polarization, since it defines the functionality of the organelles. The oligomycin ‘null-point’ assay can be used under two different loading conditions. If the loading is sufficient for aggregation of the probe in the matrix and consequent fluorescence quenching, then mitochondrial hyperpolarization will be reflected in a decreased whole-cell fluorescence as cytoplasmic probe is accumulated into the quenching environment of the matrix.21 Conversely depolarization will cause an elevation of signal as probe is released back into the cytoplasm before re-equilibrating across the plasma membrane. These effects are demonstrated in Figure 3 for GT1-7puro and GT1-7bcl-2 cells. Figure 3A,B show that oligomycin causes similar hyperpolarizing effects on puro and bcl2 cells. Thus both cells with control and bcl-2 constructs maintain a sufficiently high membrane potential for net ATP synthesis. No difference in the rate of probe redistribution was detected following addition of the protonophore FCCP. Thus we cannot substantiate claims that Bcl-2 expression inhibits protonophore-induced changes in ΔΨM.20,23
Oligomycin-induced changes in ΔΨM in puro and bcl-2 cells: effect of respiratory chain inhibition. GT1-7puro or GT1-7bcl-2 cells were loaded with 50 nM TMRM+. Where indicated, 5 μg/ml oligomycin (oligo) 2.5 μM FCCP, 2 μM rotenone (rot) or 2 μM antimycin A (AA) were added. Each trace is from a single cell soma representative of at least 15 cells from three independent experiments
In order to model the change in whole-cell fluorescence which would occur upon oligomycin addition if ΔΨM were being maintained by ATP synthase reversal, puro and bcl-2 cells were treated with rotenone, which causes maximally about 90% inhibition of complex I. Such respiratory chain inhibition induces ATP synthase reversal, which limits the extent of mitochondrial depolarization. Thus Figure 3C,D show the very slight mitochondrial dequenching observed under these conditions in, respectively, puro and bcl-2 cells. The further addition of oligomycin now causes depolarization and hence further dequenching (Figure 3C,D). Antimycin A, which totally inhibits complex III causes further depolarization and the decay of ΔΨM is accelerated by the protonophore FCCP.
We were unable to obtain convincing evidence of oligomycin-induced mitochondrial depolarization within STS-treated puro cells using the above aggregation-dependent assay in which mitochondrial depolarization should generate a transient increase in whole-cell fluorescence (data not shown). Experiments were therefore repeated at confocal resolution with GT1-7 cell equilibrated with 10 nM TMRM+, a low, non-quenching concentration (Figure 4). Figure 4A shows a representative pair of control puro cells. Mitochondrial fluorescence is retained for at least 15 min in the presence of oligomycin but is rapidly dissipated by FCCP. Figure 5A shows the time-course of the integrated whole cell fluorescence from representative non-exposed puro cells in response to oligomycin and FCCP. The slight increase in fluorescence under these non-quenching conditions reflects a mitochondrial hyperpolarization as probe accumulates into the mitochondrion and the cytoplasm restores the Nernst equilibrium across the plasma membrane.21 The histograms show the mean responses from three independent experiments.
Oligomycin depolarizes STS-treated puro but not bcl-2 cells. GT1-7puro or bcl-2 cells were incubated in the absence or presence of 0.5 μM STS for 15 h. Cells were then loaded with 10 nM TMRM+ for 30 min, washed and analyzed by confocal microscopy in the presence of TMRM+. Fluorescence was monitored before and after exposure of cells to 5 μg/ml oligomycin for 5 (O5′), 10 (O10′) or 15 (O15′) min, and 2 min after addition of 2.5 μM FCCP (F2′). Note the mitochondrial depolarization upon addition of oligomycin in STS-treated puro cell that retained TMRM+. Images are representative from at least five independent experiments
Oligomycin depolarizes STS-treated puro but not bcl-2 cells. GT1-7puro or bcl-2 cells were incubated in the absence or presence of 0.5 μM STS for 15 h, equilibrated with 10 nM TMRM+ and exposed to oligomycin (O) and/or FCCP (F) as described in Figure 4 legend. Single-cell fluorescence intensity was monitored in the confocal microscope. The histograms represent the means and S.E.M. of 29–56 cells from at least three independent experiments
Figure 4B shows three representative puro cells after 15 h exposure to STS. Note that only one of the cells retains mitochondrial TMRM+. Upon addition of oligomycin the mitochondrial fluorescence starts to decay and is almost undetectable after 15 min, indicating that ΔΨM was being previously maintained by ATP synthase reversal utilizing cytoplasmic ATP. This oligomycin-induced depolarisation is apparent in the time-course and mean results (Figure 5B). Analysis of fluorescence was performed only with the sub-set of cells retaining TMRM+ fluorescence after treatment with STS.
The mitochondria of the STS-exposed bcl-2-cells as a whole retain the ability to generate ATP and hyperpolarize on addition of oligomycin (Figure 5D). Interestingly, a minority of cells still depolarize following addition of the inhibitor, thus Figure 4D shows a pair of cells one of which retains potential and one of which shows a decay of potential after oligomycin.
Cell respiration
To further investigate the mean bioenergetic state of the mitochondrial population in STS-exposed cells, respiration of intact cells was determined (Table 1). In the absence of STS the rates of oxygen consumption in control GT1-7puro cells were not statistically different from those in GT1-7bcl-2 cells. However, the endogenous inner membrane proton leak of the in situ mitochondria, monitored by respiration in the presence of oligomycin, was increased by STS by 92% in puro cells, but by only 23% in bcl-2 cells.
Furthermore, oligomycin failed significantly to inhibit respiration in these cells, which could indicate that the mitochondria were failing to generate ATP or that substrate supply and/or electron transport were limiting respiration. The latter is confirmed by the marginal stimulation of respiration on addition of protonophore to STS-treated puro cells (Table 1) and the observation that STS decreased respiratory capacity, in the presence of protonophore, by 31% in puro cells and by 20% in bcl-2 cells. The lack of respiratory control of the mitochondria in the puro cells is a further indication of a sub-optimal ΔΨM in the mitochondrial population.
Discussion
Much of the prolonged controversy on the relationship between the time-course of mitochondrial polarization, cyt c release and apoptosis has stemmed from the inappropriate use of cationic membrane-permeant fluorescent dyes to monitor ΔΨM. As demonstrated in Figure 6C,E, diametrically opposite responses are obtained if the dyes are loaded into cells at concentrations sufficient to exceed matrix concentrations at which dye aggregation and fluorescent quenching occurs.5 We have previously presented a program in Microsoft Excel that uses simple, verifiable assumptions to model the fluorescence response of single cells equilibrated with potentiometric probes.21 These assumptions are that probes are non-selectively permeable across plasma and mitochondrial membranes, that they seek to equilibrate towards a Nernst equilibrium across both membranes, that the half-time for equilibration across the inner mitochondrial membrane is much faster than across the plasma membrane due to the greater surface/volume ratio of the former, and finally that matrix fluorescence is proportional to probe concentration until the threshold for formation of the non-fluorescent dye aggregate is reached.
Simulation of single-cell and in situ mitochondrial fluorescence in response to changes in mitochondrial membrane potential associated with oligomycin addition. The simulation is detailed in23 and is available on-line at http://www.buckinstitute.org/nicholls.htm. The following parameters were employed: plasma membrane potential, −60 mV (constant); starting ΔΨM, −150 mV; matrix volume relative to cytoplasm, 1%; plasma membrane rate constant, 0.02 s−1; TMRM+ concentration 10 nM (C, D) or 50 nM (E); matrix quench threshold 60 μM. Addition of oligomycin ‘hyperpolarized’ cells by 10 mV or caused a linear depolarization of 1 mV/min. Compare trace C with Figure 5A, C or D; compare trace D with Figure 5B and compare trace E with Figure 3A,B
The response of representative control puro and bcl2 cells to the sequential additions of oligomycin and FCCP under quenching conditions (50 nM TMRM+, Figure 3A,B) or non-quenching conditions (10 nM TMRM+, Figure 5A,C) can be modeled by a 10 mV hyperpolarization upon oligomycin addition and a total collapse of ΔΨM with FCCP (Figure 6A). The simulation under non-quenching conditions (Figure 6C) closely resembles the experimental traces in Figure 5A,C,D. It should be noted that this simulation is transformed into the corresponding simulated trace for matrix quenching conditions (Figure 6e) merely by increasing the ‘extracellular’ probe concentration parameter in the simulation from 10 to 50 nM. This simulation now closely resembles the experimental traces obtained in the non-confocal single-cell fluorescence traces reported in Figure 3A,B for puro and bcl-2 cells not exposed to STS.
The decrease in fluorescence of the STS-exposed puro cells equilibrated with low TMRM+ following oligomycin addition is more difficult to model accurately. However the time course of the decrease, and the insensitivity to subsequent addition of FCCP, can be reproduced by a linear mitochondrial depolarization at 1 mV/min following oligomycin addition. This solution to the simulation is not intended to be unique, but rather to illustrate the response to an oligomycin-induced decay of a ΔΨM that had been maintained by ATP hydrolysis.
There is clearly no single mechanism for the release of cyt c from mitochondria. Heiskanen et al.8 observed a parallel mitochondrial depolarization and release from mitochondria of green-fluorescent-protein conjugated cyt c in STS-treated PC6 cells. However, other reports have shown that cyt c release can occur before major changes in ΔΨM, suggesting that mitochondrial depolarization occurs independently and is not a factor required for the release of cyt c.9,11,24 The BH3-only proteins, such as Bid and Bad, and unlike Bax/Bak, were shown to induce cyt c release without changes in ΔΨM or the opening of the permeability transition pore and without interacting with VDAC.25 In all these studies it is of course essential to confirm that the fluorescence changes are being accurately reported. The loading concentration of probe is particularly important. In the GT1-7 cells, equilibration with 50 nM TMRM+ exceeds the quench threshold and results in a decreased whole-cell signal in response to mitochondrial hyperpolarization (see Figures 3A and 6E) whereas 10 nM probe shows a slow increase in signal under the same conditions (see Figures 5A and 6C). The appropriate concentration is particularly vital where flow cytometry is employed to monitor potential. As reported by Rottenberg and Wu26 exceeding the quench threshold means that the whole-cell signal becomes insensitive to changes in ΔΨM, and this can be validated in the ‘virtual cell’ simulation.21 The threshold for rhodamine 123 quenching is passed when loading concentrations are in the nM range;26 thus studies such as those of VanderHeiden et al.6 which loaded cells with up to 13 μM rhodamine 123 prior to flow cytometry can require re-interpretation.
Cyt c release in the absence of mitochondrial depolarization has been ascribed to a transient opening of the permeability transition pore.27 Our data suggest that cells can maintain ΔΨM following cyt c release by ATP hydrolysis. The decrease in state 3 respiration upon incubation with STS (Table 1) may account for the inhibition of electron transport and compromised respiratory function, which may form the basis for the hydrolysis of ATP. A decrease in state 3 respiration may be due to oxidative damage of the inner mitochondrial membrane or the mitochondrial complexes, which inhibits the electron transport and may increase membrane leakiness (producing the enhanced state 4 respiration, Table 1). Bcl-2 has been reported to prevent changes in ΔΨM associated with protonophore-induced apoptosis in PC12 cells23 or to prevent protonophores from depolarizing mitochondria from Bcl-2 expressing cells.20 However, our data indicates that FCCP mediates depolarization as effectively in bcl-2 cells as in puro cells (Figure 3).
Materials and Methods
Materials
GT1-7 cells transfected with a recombinant retrovirus carrying bcl-2 and a puromycin resistance gene (GT1-7bcl-2) and a control construct (GT1-7puro) were kindly donated by Dr. Bredesen (Buck Institute, CA, USA). Tetramethylrhodamine methyl ester (TMRM+), fura-2 acetoxymethyl ester (Fura-2/AM), MitoTracker-Green, SYTO-13 and propidium iodide were obtained from Molecular Probes (Leiden, Netherlands). Annexin V-FITC apoptosis detection kit and STS were from Calbiochem-Novabiochem Ltd. Nottingham, UK). Cyt c antibodies, against native and denatured forms, were obtained from PharMingen (San Diego, CA, USA). Dulbecco's modified Eagle's medium (DMEM), Hank's balanced salt solution (HBSS) and Trypsin-EDTA were from Gibco-BRL (Paisley, Strathclyde, UK). Poly-L-lysine (70–150 kDa), fetal bovine serum, penicillin/streptomycin and anti-IgG antibodies conjugated with TRITC or FITC were obtained from Sigma Chemical Co. (Poole, Dorset, UK).
Culture of GT1-7 cells (puro and bcl-2 transfected)
GT1-7 cells, a hypothalamic neuron cell-derived line (GT1-7puro cells)28 and bcl-2 transfected cells (GT1-7bcl-2 cells), as previously verified by Mah et al.17 or Kane et al.,29 were maintained in DMEM containing 25 mM D-glucose, supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin on poly-L-lysine coated plates, in an atmosphere of 5% CO2/95% air, at 37°C. The medium was changed every 3 days. One day before the experiments, the cells were harvested by trypsinization (0.05% Trypsin-0.53 mM EDTA in HBSS without Ca2+ and Mg2+) and plated at a density of 0.04×106 cells/cm2 in 22 mm square coverslips for confocal imaging, at a density of 0.08×106 cells/cm2 in 13 mm round coverslips for epifluorescence imaging, at a density of 0.24×106 cells/cm2 in 60 mm dishes for intact cell respiration and at a density of 0.7–0.9×106 cells/cm2 in 100 mm dishes for mitochondrial isolation and Western blotting analysis.
Induction of apoptosis and incubation of GT1-7 cells
Apoptotic cell death was induced by treating the cells with the protein kinase inhibitor STS (500 nM), in complete DMEM, for 1–18 h. After the treatment, the cells were rapidly and gently washed and incubated with Krebs-Ringer solution (basal medium), containing (in mM): 127 NaCl, 5.5 KCl, 2 MgSO4, 2 CaCl2, 0.5 KH2PO4, 20 HEPES, 10 glucose, pH 7.4, at 37°C. Where otherwise indicated, the cells were exposed to 5 μg/ml oligomycin and 2.5 μM FCCP.
Immunocytochemistry for detection of cyt c release from the mitochondria
Cells grown on 22-mm square coverslips were washed in basal medium and incubated with 750 nM MitoTracker-Green (in basal medium) for 60 min, at 37°C. Cells were then washed with phosphate-buffered saline solution (PBS) and fixed using freshly prepared 3.5% paraformaldehyde in HBSS, pH 7.4, for 15 min at room temperature. The cultures were washed in PBS, incubated with 20 mM glycine/PBS, for 15 min, and permeabilized with freshly prepared 0.1% saponin/PBS, for 30 min, at room temperature. The cells were incubated with a purified mouse anti-cyt c monoclonal antibody (PharMingen) that recognizes the native form of cyt c (1 : 100, diluted in 0.1% saponin/PBS) for 30 min at room temperature. The cells were washed and further incubated with a secondary anti-mouse IgG antibody conjugated with TRITC (1 : 50, diluted in 0.1% saponin/PBS) for 30 min, at room temperature. Coverslips were prepared with mounting medium for fluorescence on a microscope slide and examined by confocal microscopy.
Single cell epifluorescence imaging
Mitochondrial membrane potential was monitored with tetramethylrhodamine methyl ester at a loading concentration sufficient to cause dye aggregation within the mitochondrial matrix.5 For combined TMRM+ and fura-2 imaging, the cells, cultured on round coverslips, were loaded with 30 μM fura-2/AM and 50 nM TMRM+ for 30 min at 37°C, in basal medium containing 30 μg/ml bovine serum albumin and 0.003% pluronic acid. After washing, single cell fluorescence was monitored in the presence of TMRM+ by triple-wavelength excitation at 340/380/488 nm with emission at >510 nm, using a Nikon DIAPHOT-TMD inverted epifluorescence microscope equipped with a 40× oil immersion objective and Sutter filter wheel. Processing of fluorescence images was performed using a MiraCal Imaging facility (Life Science Resources, Cambridge, UK).
Confocal imaging
GT1-7 cells cultured on 22 mm square coverslips were loaded with 10 nM TMRM+, a concentration insufficient for matrix aggregation5 for 30 min (37°C) and mounted in a Warner RC-21B closed bath chamber on a PH-2 heater platform. The images were collected on a Zeiss LSM-510 laser scanning confocal microscope. TMRM + fluorescence was monitored in basal medium containing 10 nM TMRM+ by excitation at 543 nm and emission at >585 nm. Images were collected each 30 s.
In order to visualize cyt c in cells that had been labeled with TMRM+, immunofluorescence was performed on the confocal stage to retain the same field, in a similar way as described above, except that the secondary antibody was FITC-conjugated (anti-mouse IgG, 1 : 50). Cyt c was detected by excitation at 488 nm and emission at 505–550 nm. Visualization of both mitochondria and cyt c in mounted coverslips was performed by dual excitation at 488 and 543 nm. Cyt c was visualized at >585 nm and MitoTracker-green at 505–530 nm.
Apoptotic nuclei were visualized following incubation with 500 nM SYTO-13 (ex 488 nm, em 505–530 nm) and 1 μM propidium iodide (ex 543 nm, em 584–615 nm) in basal medium for 5 min at 37°C.
Externalized phosphatidylserine was monitored (ex 488 nm, em 505–530 nm) with An Annexin V-FITC apoptosis detection kit (CalBiochem). Propidium iodide (ex 543, em >585 nm) was used to exclude necrotic or late apoptotic cells.
Intact cell respiration
Rates of cellular respiration were measured in trypsinized cells, after centrifugation (1000 r.p.m. for 5 min) in complete DMEM to inhibit further tryptic activity. Cells (5×106 in 1 ml) were resuspended in basal medium containing glucose and placed in a thermostatically controlled (37°C) oxygen electrode chamber (Oxytherm, Hansathec Instruments Ltd., Norfolk, UK). State 4 respiration was measured in the presence of 5 μgml oligomycin and uncontrolled respiration following addition of 2.5 μM FCCP.
Mitochondrial isolation from GT1-7 cells
After incubation with STS, cells were rinsed twice with ice-cold phosphate-buffered saline and scraped into 1 ml ice-cold isolation-buffer: 250 mM sucrose, 20 mM HEPES-KOH (pH 7.4), 1 mM Na-EGTA and 1 mM Na-EDTA, with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride and 1 : 100 protease inhibitor cocktail (Sigma), containing 4-(2-aminoethyl)benzenesulfonylfluoride, pepstatin A, trans-epoxysuccinyl-L-leucylamido (4-guanidino) butane (E-64), bestatin, leupeptin and aprotinin, added just before the experiment. Cells were homogenized with 20 strokes of a Dounce homogenizer at 0°C. Nuclei and intact cells were removed by centrifugation at 500×g for 12 min, at 4°C. An aliquot of the resulting supernatant (S1) was stored at −80°C, whereas the rest was subjected to centrifugation at 9500×g for 10 min at 4°C, to pellet the mitochondria. The mitochondrial pellet was washed once with the isolation-buffer and centrifuged at 9500×g for 10 min at 4°C. The resulting pellet was resuspended in the isolation-buffer and frozen at −80°C. The protein concentration in the samples was determined by the BioRad protein assay.
Cyt c and Bcl-2 by Western blotting
Equivalent amounts of protein from the S1-supernatant or from the mitochondrial fractions were resolved on 12% SDS–PAGE, after denaturation at 100°C for 5 min in buffer containing 100 mM Tris-HCl, pH 6.8, 200 mM dithiothreitol (DTT), 4% SDS, 0.2% bromophenol blue and 20% glycerol. The proteins were transferred onto nitrocellulose (ImmobilonTM-P, Millipore) which were incubated overnight at 0–4°C in blocking buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl and 0.05% Tween-20) containing 5% BSA. Blots were then incubated with mouse anti-cytochrome c antibody (PharMingen) against the denatured mitochondrial protein (1 : 1000 dilution in blocking buffer containing 1% BSA) or with mouse anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1 : 500 dilution, against the denatured S1-supernatant protein. After washing, immunoblots were incubated with horseradish peroxidase-conjugated secondary antibody (1 : 1000 dilution) and autoradiographed with enhanced chemiluminescence (Amersham Pharmacia Biotech).
Statistical analysis
Numerical data are presented as means±S.E.M. for the indicated number of experiments; for statistical analysis Student's test was used.
Abbreviations
- FCCP:
-
carbonylcyanide-p-(trifluoromethoxy) phenylhydrazone
- STS:
-
staurosporine
- ΔΨM:
-
mitochondrial membrane potential
- TMRM+:
-
tetramethylrhodamine methyl ester
- PS:
-
phosphatidylserine
- cyt c:
-
cytochrome c
References
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X . 1997 Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade Cell 91: 479–489
Jiang X, Wang X . 2000 Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1 J. Biol. Chem. 275: 31199–31203
Shimizu S, Narita M, Tsujimoto Y . 1999 Bcl-2 family of proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC Nature 399: 483–487
Crompton M . 2000 Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis Curr. Opin. Cell Biol. 12: 414–419
Nicholls DG, Ward MW . 2000 Mitochondrial membrane potential and cell death: mortality and millivolts Trends Neurosci. 23: 166–174
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB . 1997 Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria Cell 91: 627–637
Prehn JHM, Jordán J, Ghadge GD, Preis E, Galindo MF, Roos RP, Krieglstein J, Miller RJ . 1997 Ca2+ and reactive oxygen species in staurosporine-induced neuronal apoptosis J. Neurochem. 68: 1679–1685
Heiskanen KM, Bhat MB, Wang HW, Ma JJ, Nieminen AL . 1999 Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells J. Biol. Chem. 274: 5654–5658
Finucane DM, Waterhouse NJ, Amarante-Mendes GP, Cotter TG, Green DR . 1999 Collapse of the inner mitochondrial transmembrane potential is not required for apoptosis of HL60 cells Exp. Cell Res. 251: 166–174
Budd SL, Tenneti L, Lishnak T, Lipton SA . 2000 Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons Proc. Natl. Acad. Sci. USA 97: 6161–6166
Krohn AJ, Wahlbrink T, Prehn JHM . 1999 Mitochondrial depolarization is not required for neuronal apoptosis J. Neurosci. 19: 7394–7404
Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Örd T, Bredesen DE . 1993 Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species Science 262: 1274–1277
Sarafian TA, Vartavarian L, Kane DJ, Bredesen DE, Verity MA . 1994 Bcl-2 expression decreases methyl mercury-induced free-radical generation and cell killing in a neural cell line Toxicol. Lett. 74: 149–155
Wiedau-Pazos M, Trudell JR, Altenbach C, Kane DJ, Hubbell WL, Bredesen DE . 1996 Expression of Bcl-2 inhibits cellular radical generation Free Radic. Res. 24: 205–212
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X . 1997 Prevention of apoptosis by bcl-2: release of cytochrome c from mitochondria blocked Science 275: 1129–1132
Srinivasan A, Foster LM, Testa M-P, Örd T, Keane RW, Bredesen DE, Kayalar C . 1996 Bcl-2 expression in neural cells blocks activation of ICE/CED-3 family proteases during apoptosis J. Neurosci. 15: 5654–5660
Mah SP, Zhong LT, Liu Y, Roghani A, Edwards RH, Bredesen DE . 1993 The protooncogene bcl-2 inhibits apoptosis in PC12 cells J. Neurochem. 60: 1183–1186
Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G . 1996 Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria Proc. Natl. Acad. Sci. USA 93: 9893–9898
Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE . 1996 Shift of the cellular oxidation-reduction potential in neural cells expressing bcl-2 J. Neurochem. 67: 1259–1267
Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y . 1998 Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux Proc. Natl. Acad. Sci. USA 95: 1455–1459
Ward MW, Rego AC, Frenguelli BG, Nicholls DG . 2000 Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells J. Neurosci. 20: 7203–7219
Rego AC, Ward MW, Nicholls DG . 2001 Mitochondria control AMPA/kainate receptor-induced cytoplasmic calcium deregulation in rat cerebellar granule cells J. Neurosci. 21: 1893–1901
Dispersyn G, Nuydens R, Connors R, Borgers M, Geerts H . 1999 Bcl-2 protects against FCCP-induced apoptosis and mitochondrial membrane potential depolarization in PC12 cells Biochim. Biophys. Acta 1428: 357–371
Bossy-Wetzel E, Newmeyer DD, Green DR . 1998 Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization EMBO J. 17: 37–49
Shimizu S, Tsujimoto Y . 2000 Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity Proc. Natl. Acad. Sci. USA 97: 577–582
Rottenberg H, Wu SL . 1998 Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells Biochim. Biophys. Acta 1404: 393–404
Pastorino JG, Tafani M, Rothman RJ, Marcineviciute A, Hoek JB, Farber JL . 1999 Functional consequences of the sustained or transient activation of the mitochondrial permeability transition pore J. Biol. Chem. 274: 31734–31739
Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI . 1990 Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis Neuron 5: 1–10
Kane DJ, Örd T, Anton R, Bredesen DE . 1995 Expression of Bcl-2 inhibits necrotic neural cell death J. Neurosci. Res. 40: 269–275
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Rego, A., Vesce, S. & Nicholls, D. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1-7 neural cells. Cell Death Differ 8, 995–1003 (2001). https://doi.org/10.1038/sj.cdd.4400916
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400916
Keywords
This article is cited by
-
Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin
Acta Neuropathologica Communications (2018)
-
High glycolytic activity of tumor cells leads to underestimation of electron transport system capacity when mitochondrial ATP synthase is inhibited
Scientific Reports (2018)
-
Pathogenic p62/SQSTM1 mutations impair energy metabolism through limitation of mitochondrial substrates
Scientific Reports (2017)
-
Mitochondrial bioenergetics and neuronal survival modelled in primary neuronal culture and isolated nerve terminals
Journal of Bioenergetics and Biomembranes (2015)
-
Effects of transmembrane potential and pH gradient on the cytochrome c-promoted fusion of mitochondrial mimetic membranes
Journal of Bioenergetics and Biomembranes (2013)